The Late Electrophysiological Consequences Of Posterior Wall Isolation In Patients With Atrial Fibrillation
Davies EJ, Lines I, Dalrymple-Hay M, Haywood GA
South West Cardiothoracic Centre, Plymouth, PL6 8DH. UK.
There are many different lesion sets that are used for the surgical ablation of atrial fibrillation (AF). One such pattern is the ‘box set’, a single ring of scar delivered anterior to the pulmonary veins, which aims to electrically isolate the posterior wall from the rest of the heart. However it remains unclear whether posterior wall isolation (PWI) is an effective lesion set for maintenance of sinus rhythm and whether it is necessary to achieve complete bidirectional block. We investigated the long-term integrity of the ‘box set’ lesion created during surgical AF ablation by epicardial High Intensity Focussed Ultrasound (HIFU). All patients had documented persistent or recurrent paroxysmal AF prior to surgery. We correlated this with subsequent success or failure in the abolition of atrial fibrillation.
With regional ethical and R&D approval, 101 patients who had previously undergone HIFU AF ablation greater than 4 years ago were screened for inclusion in the study. 17 patients agreed to late electrophysiological study: 11 with on-going AF and 6 in normal sinus rhythm.
Clinical history and 7-day holters were used to define the NSR group. We performed a diagnostic EP study using a transseptal approach in fully anticoagulated patients (INR>2.0 and ACT maintained at >300s). A catheter was placed in the coronary sinus (CS) and a circular multipolar mapping catheter was used to map the left atrium and pulmonary veins. Patients in atrial fibrillation were cardioverted. We recorded whether posterior wall (PW) and pulmonary vein (PV) isolation had been achieved at the surgical procedure. In selected cases we recorded a voltage map using either CARTO (Biosense- Webster) or NavX (St Jude Medical) to identify areas of ablation scar.
All 11 patients with AF had absence of PW+PV isolation with fractionated electrograms recorded across the PW. In the 6 patients with long-term freedom from AF, PW+PV isolation was confirmed in 4 (67%) and in 1 there was prolonged conduction across the box-set lesion with CS to PW activation time of around 200ms versus 45ms from mid-CS to left atrial appendage. Of the 4 patients with confirmed PW+PV isolation, 1 had dissociated spontaneous atrial potentials within the box set area and the other 3 had electrical silence throughout with inability to capture the posterior wall pacing at 10mA at multiple sites.
There appears to be a clear correlation between the successful restoration of long-term sinus rhythm and isolation / delayed conduction from the pulmonary veins and posterior wall. Given the advent of hybrid atrial fibrillation ablation techniques designed to deliver this lesion set, these findings are timely and highly relevant.
Key Words : Atrial Fibrillation,, Surgical Ablation, High Intensity Focussed Ultrasound, Persistent Atrial Fibrillation, Electrophysiology, Transseptal.
Correspondence to: Dr Edward DaviesSouth West Cardiothoracic CentrePlymouthDevonPL6 8DH
The mechanism by which atrial fibrillation (AF) is initiated and sustained is complex and incompletely understood. It is clear that the majority of triggers for AF reside within the pulmonary veins (PV) but there are also influential non-pulmonary areas. These triggers are especially important for paroxysmal AF (PAF). For persistent forms of AF, a number of different mechanistic theories have been proposed involving rotors, complex fractionated atrial electrograms (CFAE) and multiple wavelets. Regardless of the mechanism underlying persistent AF (PsAF), evidence suggests that the bulk of the substrate for these mechanisms resides in the posterior wall (PW) of the left atrium (LA).1,2
Consistent with these different theories, a wide variety of different ablation techniques are currently being practiced across international centres. The majority of operators rely on a stepwise approach involving pulmonary vein isolation (PVI) followed by further substrate modification. These techniques are sometimes personalised to the individual case.
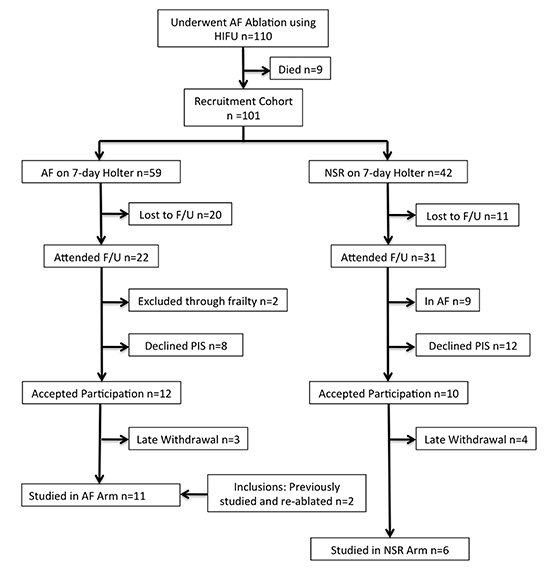
Figure 1. Flow diagram illustrating the recruitment process.
Alternative ablation techniques rely on the identification and successful ablation of CFAEs, ganglionic plexi (GP), or more novel techniques involved the mapping and ablation of putative rotors.3 These techniques are all highly complex and rely on the skill of the operator to be able to identify all appropriate electrophysiological landmarks.
An entirely different strategy is to accept the assumption that the bulk of the substrate on which AF propagates resides within the posterior left atrium. Several groups are investigating the applicability of an empirical ablation strategy that results in posterior wall isolation.4 There is evidence to suggest that such an end-point may be sufficient in maintaining sinus rhythm in the majority of patients without the complexities involved in other approaches.5 Such techniques are already being utilised; one such involves a single ring of ablation scar being delivered anterior to the pulmonary veins with the intention of electrically isolating this entire posterior wall/ pulmonary vein area. This is commonly referred to as the ‘box-set’ and may be delivered epicardially6 and/or endocardially.7
It is unclear whether freedom from AF in patients who have undergone AF ablation using the box-set pattern results from extensive debulking of the LA muscle; GP alteration / destruction; pulmonary vein isolation or from isolation of the entire PW and PV substrate.
We wished to determine whether the success of the box-set pattern was due to complete electrical isolation of the PW. One possible model to investigate this was to study the group of patients who had previously undergone AF ablation using epicardial box-set lesions delivered using High Intensity Focussed Ultrasound (HIFU).8 The aim of this study was to characterise the very-late electrical properties of patients in whom the ablation procedure had been a success and compare them to others with on-going AF. We sought to recruit 10 patients from each group for a diagnostic transseptal electrophysiological study. This study was performed in accordance with the Declaration of Helsinki and with the approval of the institutional research and development department and of the West of Scotland Regional Ethics Committee. This permission extended to the diagnostic study only; any subsequent ablation procedures were performed as ‘usual care’ with fully informed written consent being gained prior to the study.
From the original cohort of 110, 101 patients who had previously undergone HIFU AF ablation greater than 4 years ago were screened for inclusion in the study. 17 patients agreed to late electrophysiological study, Electrophysiological data from a further 2 patients who were previously ablated for on-going AF were also included. The studies were conducted at a mean of 65 months post-surgical HIFU isolation procedure. 14 underwent HIFU concomitantly with cardiac surgery, 3 had surgical AF ablation for lone AF and have previously been described in full.8
At routine follow up, patients were introduced to the study and invited to participate (EJD). Those who expressed an interest were given a patient information sheet (PIS) and were contacted by telephone to confirm willingness to participate 2-days later. Normal sinus rhythm (NSR) was confirmed by the absence of symptoms, a 7-day full disclosure ambulatory electrocardiogram (ECG) confirming absence of AF and a 12-lead ECG at time of recruitment. The AF group contained those patients having any documented recurrence of AF following a 3-month blanking-period post ablation. Patients with recurrences solely of atrial flutter (AFl) were included in the NSR group but described separately. The patient demographics (as at time of epicardial AF ablation) are shown in Table 1.
Table 1. Study participant demographics and operative data
| Participant Number |
Pre-op AF category |
Pre-op AF duration (months) |
Pre-op DCCV |
Pre-op AAD failed |
Operation |
Op to EP duration (months) |
LA size (AP) / (cm) |
EF (%) |
NTH |
CAD |
Post-op rhythm |
| 01 |
LSP |
14 |
1 |
Nil |
CABG |
60 |
4.0 |
20 |
- |
Yes |
NSR |
| 02 |
LSP |
<36 |
0 |
Nil |
MVR |
54 |
4.6 |
60 |
- |
- |
NSR |
| 03 |
LSP |
30 |
0 |
Flecanide |
MV Repair |
96 |
5.2 |
70 |
- |
- |
AF |
| 04 |
LSP |
30 |
1 |
Nil |
AVR/MVR |
82 |
2.8 |
60 |
- |
- |
NSR |
| 05 |
PAF |
24 |
0 |
Nil |
CABG |
84 |
4.6 |
45 |
Yes |
Yes |
PAF |
| 06 |
PsAF |
6 |
1 |
Nil |
CABG/ AVR |
52 |
3.8 |
45 |
- |
Yes |
NSR |
| 07 |
LSP |
50 |
0 |
Flecanide, Sotalol, Amiodarone |
Lone |
56 |
4.2 |
58 |
- |
- |
NSR |
| 08 |
LSP |
14 |
1 |
Nil |
MV Repair |
70 |
5.3 |
52 |
- |
- |
NSR |
| 9 |
PsAF |
5 |
0 |
Nil |
CABG |
63 |
5.1 |
35 |
- |
Yes |
NSR |
| 10 |
LSP |
15 |
1 |
Amio |
CABG |
71 |
5.1 |
50 |
Yes |
Yes |
AF |
| 11 |
LSP |
60 |
1 |
Nil |
MV Repair |
51 |
6.2 |
60 |
Yes |
Yes |
AF |
| 12 |
LSP |
54 |
1 |
Nil |
Lone |
17 |
5.1 |
60 |
- |
- |
AF |
| 13 |
LSP |
10 |
0 |
Nil |
AVR |
98 |
3.8 |
45 |
- |
- |
NSR |
| 14 |
LSP |
48 |
2 |
Amio |
Lone |
13 |
5.4 |
30 |
- |
- |
AF |
| 15 |
LSP |
144 |
0 |
Nil |
MVR |
83 |
5.2 |
55 |
- |
- |
AF |
| 16 |
LSP |
18 |
0 |
Nil |
MVR |
60 |
3.8 |
60 |
- |
- |
NSR |
| 17 |
LSP |
24 |
3 |
Sotalol |
CABG |
43 |
4.2 |
60 |
Yes |
Yes |
NSR |
Pre-op = Preoperative; AF = Atrial Fibrillation; LSP = Longstanding Persistent Atrial Fibrillation’ PsAF = Persistent Atrial Fibrillation; AAD = Antiarrhythmic drug; LA = Left Atrial, AP = Anterioposterior; cm = centimetre; EF = Ejection Fraction; DM = Diabetes Melltus; HTN = Hypertension; CVE = Cerebrovascular Event; BMI = Body Mass Index; CAD = Coronary Artery Disease; CTI = Carvotricuspid Isthmus Ablation; DCCV = DC Cardioversion
A transseptal electrophysiological study was performed in all patients. Patients with a CHA2DS2-VASc of <1 and on-going atrial arrhythmia were anticoagulated with either warfarin (target INR 2.0-3.0) or dabigatran (110mg bd) for a minimum of 3 weeks prior to the study. All diagnostic studies were performed as a day-case using IV sedation (diazemuls and diamorphine titrated to response). Access was gained via the right femoral and subclavian/internal jugular vein. A decapolar catheter was placed in the coronary sinus (CS) and a quadripolar catheter in the right ventricle. Using a standard Brockenbrough needle, a transseptal puncture was performed to allow passage of a steerable sheath - Channel, (BARD Electrophysiology) or Agilis® (St. Jude Medical) into the LA. Puncture was facilitated using a combination of contrast injection and pressure monitoring. IV heparin was administered to maintain the activated clotting time (ACT) at <300s for the duration of LA instrumentation. Pulmonary venography was performed for all PVs. A circular mapping catheter (Expandable Lasso or AFocus II, St. Jude Medical) was sequentially placed in all four PVs and flat on the PW. For patients in sinus rhythm at the time of the study, we tested for exit and entry block and recorded the conduction times between CS and PV. Those in AF at the time of the study were cardioverted (200J biphasic AP pad position) on a maximum of two occasions to allow for conduction to be assessed.
In selected cases of apparent PW isolation (defined as the absence of electrical activity, or evidence of spontaneous isolated atrial complexes), we tested for pace/detect within the PW. We passed a second catheter alongside the steerable sheath and used it alongside the mapping catheter to test for conduction contained within the isolated posterior wall.
In 7 patients with symptomatic drug refractory atrial arrhythmia, further ablation was performed, 5 cases for AF and 2 for AFl. A voltage map was produced using either CARTO (Biosense- Webster) or NavX (St Jude Medical). Using a contact force ablation catheter, additional lesions were delivered either around the pulmonary vein ostia and/or to complete the existing roof and floor lines created by the HIFU procedure. The ablation goal was validated PWI in the cases of AF recurrence.
Of the original 110 patients who underwent surgical AF ablation,
101 were still alive at screening follow-up. Nine had died in the interval:
4 from post-operative complications unrelated to the AF ablation
procedure or use of the Epicore device, two died from malignant
disease, one suffered an embolic stroke, one a traumatic intracerebral
bleed and one from end-stage heart failure.
Free From Atrial Fibrillation – NSR
Of the six patients who had an absence of AF, only 3 were completely
free from any atrial arrhythmia recurrence the remaining 3 had
experienced episodes of documented atrial flutter. Of the 3 patients
where no atrial arrhythmia had occurred since surgical ablation, two
had originally experienced PsAF pre-operatively (cases 6&9) and the
other infrequent PAF pre-operatively only (case 5).
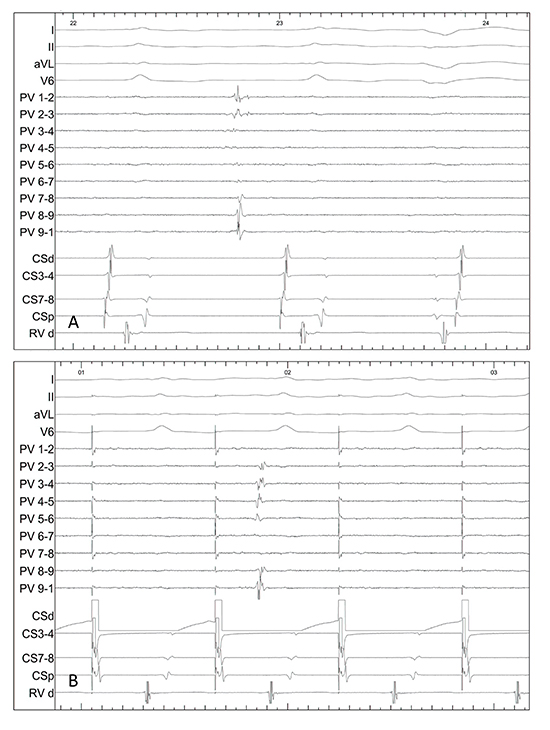
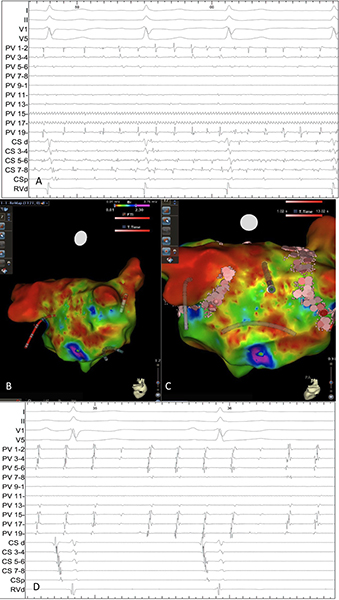
In cases 6&9, there was no conduction into or out of the PW during capture pacing from the CS together with isolated atrial potentials throughout the pulmonary veins and the posterior wall (Figure 2).
Figure 2. (A) With the circular mapping catheter (PV) placed flat on the posterior wall, an isolated premature atrial beat is seen showing electrical isolation of this region during sinus rhythm. (B) Circular mapping catheter (PV) in the right upper pulmonary vein and capture pacing from the coronary sinus, there is no evidence of conduction into the vein. Also seen is a non-conducted pulmonary vein potential (case 9).
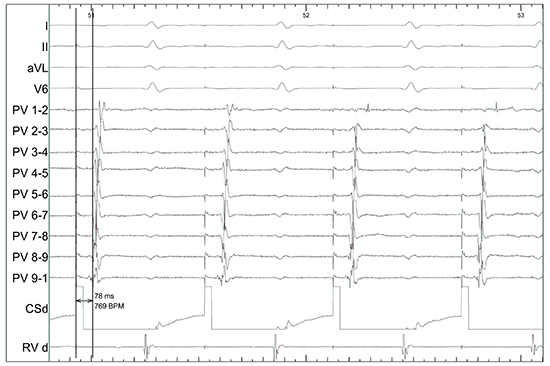
In the patient with prior pre-operative infrequent PAF (case 5), there was no evidence of either exit or entry block from the posterior region (Figure 3).
Figure 3. In a patient with preoperative paroxysmal AF and freedom from any recurrent atrial arrhythmia. Pacing from CS distal demonstrates intact conduction between the coronary sinus and the posterior wall with a conduction time of 78ms (case 5).
Free From Atrial Fibrillation – Atrial Flutter
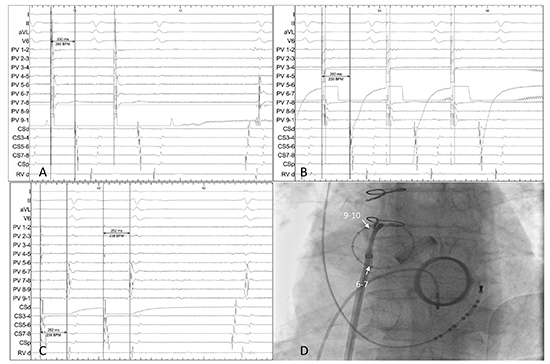
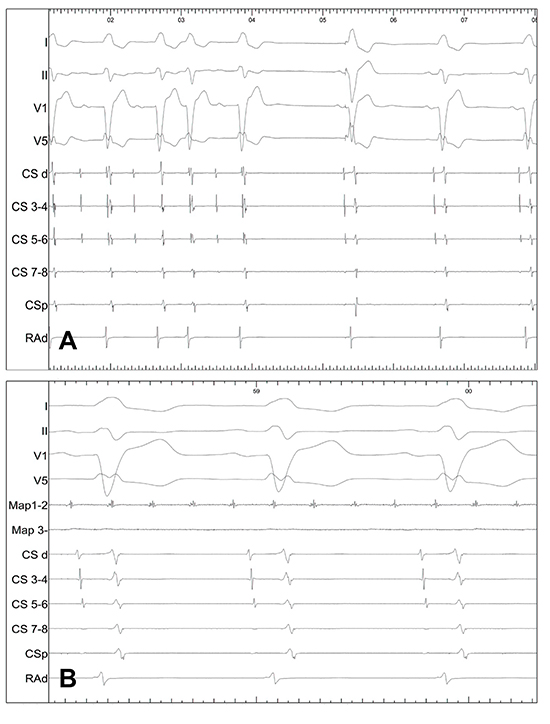
Of the 6 patients with freedom from AF, 3 have experienced documented recurrences of atypical paroxysmal atrial flutter. In 2 cases, the PW was found to be silent with intact block throughout the veins and posterior LA. The other one case had very delayed conduction between the PW and the CS (case 4). The area of conduction gap is identified near the RUPV (Figure 4)
Figure 4. In a patient with recurrence of paroxysmal left atrial flutter but freedom from atrial fibrillation. (A) Pacing from9-10 has a conduction time to CS of 230ms (B) whereas6-7 to CS is 260ms. (C) Earliest signal from CS pacing is seen in.9-10 (D) Fluoroscopy showing the circular mapping catheter on the posterior wall. The gap is therefore localised to the roof of the LA (case 4).
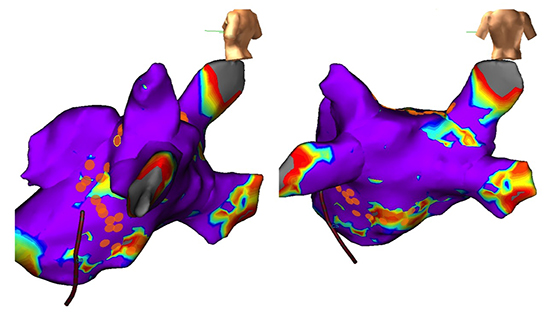
In one of the cases of post-operative AFl, the patients remained symptomatic and we proceeded on to ablate a peri-mitral flutter circuit (case 16).
Figure 5. Mitral valve replacement, pre-operative longstanding persistent atrial fibrillation with symptomatic paroxysms of an atypical atrial flutter. (A) NavX voltage map illustrates the border of the scar delivered by the HIFU device and the electrically silent posterior wall. (B) Surface marking show density of recordings over the posterior wall. (C) A peri-mitral flutter was entrainment and ablated back to sinus rhythm by joining the mitral annulus to the adjacent inferior border of the HIFU scar. At 6-month follow-up, the patient has experienced no further arrhythmia (case 16).
We studied a total of eleven participants with AF following the ablation procedure.
In all instances, persistent atrial electrograms were present throughout the PW and pulmonary veins, ranging from grade 1 to 5 in degree of fractionation.9 Cardioversion was performed to restore NSR and allow for the conduction between the CS and the PW/PV to be assessed; rapid reversion back to AF limited assessment in 7 cases.7,10-15 Where tested, the conduction times between the PW/PVs and the CS are shown in Table 2. There were no instances of conduction bock in any of the persisting AF cases.
Figure 6. Aortic valve replacement and pre-operative long-standing persistent atrial fibrillation, on-going symptomatic paroxysms of atrial fibrillation. The circular mapping catheter is on the posterior wall. Capture pacing from the coronary sinus shows rapid conduction to the posterior wall around 40ms.
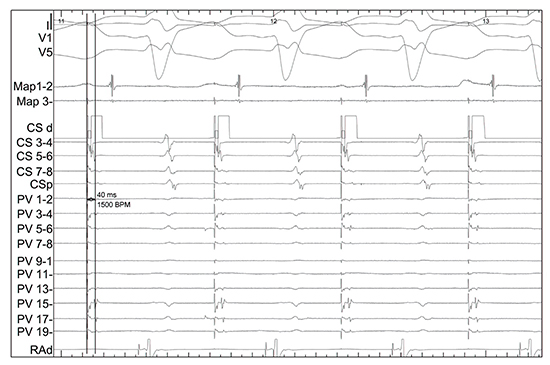
Table 2. Conduction times expressed as exit and entry between the pulmonary veins / posterior wall and coronary sinus
| CASE |
LUPV |
LLPV |
RUPV |
RLPV |
PW |
Arrhythmia following HIFU |
Additional Ablation at EP study? |
Endocardial ablation strategy |
PWI achieved? |
| Exit |
Entry |
Exit |
Entry |
Exit |
Entry |
Exit |
Entry |
Exit |
Entry |
| 01 |
192 |
194 |
200 |
190 |
168 |
166 |
166 |
172 |
190 |
226 |
AF |
No |
N/A |
N/A |
| 02 |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Paroxysmal Flutter |
No |
N/A |
N/A |
| 03 |
80 |
128 |
No capture |
54 |
152 |
126 |
106 |
132 |
108 |
106 |
AF |
Yes |
Linear ablation to PW |
No |
| 04 |
270 |
226 |
196 |
192 |
272 |
304 |
220 |
284 |
260 |
252 |
Flutter |
No |
N/A |
N/A |
| 05 |
102 |
92 |
No capture (no muscle) |
|
176 |
118 |
No capture (no muscle) |
|
98 |
98 |
NSR |
No |
N/A |
N/A |
| 06 |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
NSR |
No |
N/A |
N/A |
| 07 |
Rapid reversion to AF following DCCV – proceeded onto additional ablation |
|
|
|
|
|
|
|
|
|
AF |
Yes |
Bilateral WACA and roof line to join scar on floor |
Yes |
| 08 |
No capture (no muscle) |
|
|
|
|
|
|
|
136 |
104 |
AF |
Yes |
Roof and floor line and linear to PW |
Yes |
| 09 |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
NSR |
No |
N/A |
N/A |
| 10 |
Reversion to AF |
|
|
|
118 |
106 |
Reversion to AF |
|
|
|
AF |
No |
N/A |
N/A |
| 11 |
Reversion to AF following PW testing |
|
|
|
145 |
152 |
Reversion to AF |
|
120 |
118 |
AF |
No |
N/A |
N/A |
| 12 |
Rapid reversion to AF following DCCV – proceeded onto additional ablation |
|
|
|
|
|
|
|
|
|
AF |
Yes |
Linear ablation to posterior wall. |
No |
| 13 |
Reversion to AF following PW testing – proceeded onto additional ablation |
|
|
|
|
|
|
|
42 |
40 |
AF |
Yes |
Bilateral WACA to join HIFU roof and floor. |
Yes |
| 14 |
Rapid reversion to AF following DCCV – proceeded onto additional ablation |
|
|
|
|
|
|
|
|
|
AF |
Yes |
Linear ablation to posterior wall |
No |
| 15 |
Unsuccessful DC Cardioversion – CFAE throughout PW and PVs |
|
|
|
|
|
|
|
|
|
AF |
No |
N/A |
N/A |
| 16 |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Blocked |
Flutter |
Yes |
Yes – mitral isthmus |
N/A |
| 17 |
160 |
138 |
194 |
204 |
148 |
200 |
190 |
186 |
158 |
154 |
AF |
Yes |
Attempted completion of box set. |
No |
shaded cases indicate absence of post-operative AF). (AF = Atrial Fibrillation; DCCV = Direct Current Cardioversion; PW = Posterior wall; PV = Pulmonary Veins; CFAE = Complex Fractionated Atrial Electrogram, HIFU = High Intensity Focussed Ultrasound; WACA = Wide Area Circumfrential Ablation
In summary, of the 11 patients with post-operative AF, none showed any evidence of PW isolation. Conversely, of the 6 patients with absence of post-operative AF, 4 had electrically isolated PW, one had delayed conduction between PW and CS and one (post-operative infrequent PAF only) had intact conduction.
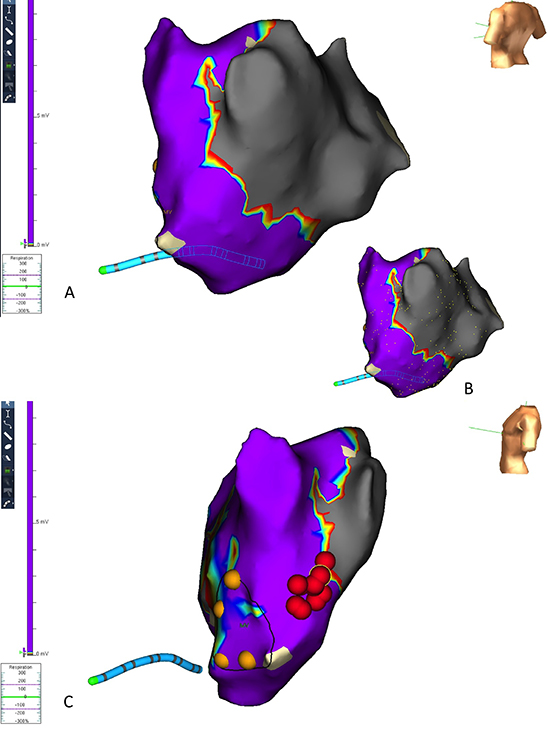
Figure 7. Coronary artery bypass grafting and pre-operative longstanding persistent atrial fibrillation; failed HIFU ablation with persistent atrial fibrillation. NavX voltage map shows minimal evidence of the transmural scar delivered by the HIFU apparatus and an electrically active posterior wall. In this image, the additional endocardial ablation points delivered to complete the box are shown as red dots and the CS catheter is shown in red (case 17).
Additional Findings During AF Ablation
During instrumentation of the LA, patient 13 developed AF with organised (grade 5) fractionated electrograms throughout the PW. Voltage mapping showed established transmural scar line along the left atrial roof and floor. We proceeded to isolate the right PV pair using a standard wide area circumferential ablation (WACA) approach resulting in rhythm organization (cycle length of 250ms). Following completion of the left PV-encircling lesion set continuous with the roof-line, spontaneous resolution of SR occurred (Figure 8A). The PW is shown to contain a flutter circuit while surface ECGs show NSR (Figure 8B). This PW block persisted during the administration of 15mg of adenosine, sufficient to caused AV block.
Figure 8. (A) Completion of the WACA lesion encircling the right sided veins to join the roof line resulted in reversion to sinus rhythm. (B) Following completion of the bilateral PV-encircling lesions joining to the HIFU roof and floor lines, sinus rhythm is seen on the surface electrodes while the PW (here demonstrated through the ablation catheter, Map 1-2) shows the PW to persist in flutter (case 13).
DC cardioversion resulted in non-sustained sinus rhythm; no conduction intervals were obtained during this short window but the circular mapping catheter showed fractionated signals (Figure 9A). A voltage map was constructed using CARTO 3 (Figure 9B); established transmural scar is evident along the floor of the left atrium but was incomplete on the roof and anterior to both pairs of pulmonary veins. Bilateral PV-encircling lesions were delivered together with the completion of a roof-line (Figure 9C) resulting in spontaneous return of sinus rhythm (Figure 9D).
Figure 9. (A) Circular mapping catheter (marked as PV) on the posterior wall showing widespread fractionated atrial electrograms. (B) Initial voltage map showing the external view of the left atrium with the left pulmonary vein pair to the right of the image. The HIFU scar (red) is demonstrated running along the floor of the left atrium. (C) Additional ablation using a SmartTouch catheter (here depicted as pink dots) to encircle the left and right pulmonary vein pairs together with a roof line is successful in restoring normal sinus rhythm. (D) The posterior wall is seen to contain a flutter circuit (through mapping catheter, PV) in the presence of sinus rhythm on the surface electrograms (case 7).
Outcome From Additional Ablation Cases
All cases who had additional ablation performed for post-operative AF are now in sinus rhythm (one on a class 3 antiarrhythmic) except one (case 17) in whom the posterior wall was not successfully isolated due to poorly tolerating the catheter ablation under sedation.
Although several groups have reported on the invasive electrophysiological
findings following ablation, to the best of our knowledge,
this is the first study aiming to investigate the association between
PW isolation and long-term (>4 years) freedom from AF. A summary
of the previous knowledge gained from such studies is provided in
Table 3, most of which is obtained from studies involving redo procedures.
As such, there is a skew against reporting what constitutes a
successful outcome.
Table 3. Summary of the previous studies reporting invasive electrophysiological findings following ablation strategies intending to achieve posterior wall isolation
| Study |
Number |
Lesion pattern / Energy |
Validated PWI? |
Follow-up findings |
Conclusions |
Complications |
| Catheter Based |
| Ernst et al.10
Case series |
13 |
RF; Box, MI. |
0% |
100% AF recurrence but no prior PWI. |
Failure to deliver intended lesion set resulted in 100% AF recurrence. |
Nil reported. |
| Tamborero et al13
RCT |
60: 35 PAF, 13 PsAF and 12 LSP AF |
RF; BiWACA, roof and floor line. |
100% |
67% of initial PWI group with recurrent arrhythmia had reconnected roof-line and electrical activity on PW. |
“LA posterior wall isolation does not improve the outcome of CPVA” |
• 1 TIA
• 1 inf. STEMI. |
| Kumagai et al15 |
91 PAF |
RF; Box. |
90% |
6 redo cases (3 AFl/AT, 3 AF); all had reconnection – none had intact PWI |
“86 (95%) of the 91 patients were free of AF, including six patients after the second procedure without antiarrhythmic drugs. The remaining five patients were arrhythmia-free but required drugs.” |
Nil reported. |
| Lim et al.12
Case series |
100: 66 PAF, 18 PsAF, 16 LSP AF. |
RF; Box ± MI ± CTI. |
96% |
“30 of 34 patients had breaches in the single ring of lesions that led to resumption of electrical activity in the previously isolated PLA.” “… none of the 4 patients who still had intact isolation of the PLA had recurrence of AF after their first procedure…” |
“Recurrence of atrial arrhythmias after single-ring isolation of the PLA and pulmonary veins is usually associated with reconnection across the ring of ablation lesions…” |
Nil reported. |
| Sanders et al.14
Case series |
27 “chronic” AF |
RF; BiWACA, roof and floor line, CTI. |
Yes |
One case of PWI with AF recurrence. |
“Isolation of the posterior-LA in patients with chronic AF is associated with prolongation of the AFCL, incremental to the effect of PV isolation, and termination of AF in ≈20%.” |
• 1 tamponade
• 1 temporary phrenic nerve injury. |
| Chen et al.21
Case series |
42: 18 PAF, 14 PsAF and 10 LSP AF |
BiWACA, roof and floor line. |
100% |
6 cases of redo for AF; all had reconnection of PW. |
“Silencing electrical activity in the PIA is feasible, and capable of decreasing the rate of AF recurrence.” |
Nil reported. |
| Surgical based |
| Todd et al.11
Case series
|
14; 11 PAF, 3 PsAF/LSP AF |
Incision and cryo; Box, MI, CTI. |
|
100% isolated at 6-day EP study. One late recurrence of AF – gap in cryo line. |
“The unique data collected from these patients support the principle of isolation of the pulmonary veins and posterior LA as a curative therapy, regardless of the method used” |
• 1 pleural effusion |
| Sueda et al.22
Case series |
49 LSP AF |
Incision and cryo/RF; |
Not stated |
30% AF recurrence. No EP findings provided. |
Nil relevant. |
Nil |
| Hybrid Based |
| Pison et al.23 |
23; 1 LSP AF, 9 PsAF, 13 PAF |
RF; BiWACA , roof and floor line |
100% |
2 patients redo: one with flutter and one AF (gap in ring) |
“A combined transvenous endocardial and thoracoscopic epicardial ablation procedure for paroxysmal and recent persistent AF resistant to AADs has a single-procedure success rate of 83% at 1 year.” |
• 1 pleural effusion
• 1 hospitalized for 13 days -chest pain at the insertion sites |
| Bisleri et al.5
Case series |
45 LSP AF |
Epicardial RF; Box |
100% exit block
91.1% entrance block |
No correlation made between rhythm and EP findings at time of second stage. |
92.6% freedom from AF in those with documented PW isolation at 28-month follow-up. |
Nil |
PAF = Paroxysmal Atrial Fibrillation; PsAF = Persistent Atrial Fibrillation; LSP AF; Long-standing Persistent Atrial Fibrillation; WACA = Wide Area Circumferential Ablation; PWI = Posterior Wall Isolation; RF = Radiofrequency; Cryo = Cryoablation; EP = Electrophysiology; Box = Box-set lesion; MI = Mitral Isthmus; CTI = Carvotricuspid Isthmus; AFl = Atrial Flutter; AT = Atrial Tachycardia
Previous Studies On The Box Set.
Possibly the first group to attempt a box pattern using catheters was Ernst et al. in 1999. The limited technology available at that time resulted in failure to achieve PWI in all of their cases.10 Todd et al. describes the initial 2003 experience following the combined open-chest surgical and catheter cryoablation box pattern for AF.11 At 6-days post procedure, all cases had persistently intact posterior wall isolation, determined through epicardial wires. Late recurrent of atrial arrhythmias in one patient resulted in EP restudy - a gap was found in the posterior wall isolation, closure of which restored sinus rhythm. Todd demonstrated that the isolated PW was able to sustain atrial arrhythmia independent of the remaining atria; this finding has been replicated in our study.
Lim et al. provides a comprehensive assessment of the mechanisms of arrhythmia recurrence following single ring ablation delivered by catheter.12 In 100 patients with an end-point of validated PWI, 69% experienced recurrence of an atrial arrhythmia (35% AF and 34% flutter). In those patients that underwent a redo procedure for recurrent AF, all had gaps in the ablation ring with conduction in and out of this region. In six of the patients, a flutter circuit was established through two gaps in the ring. Four patients had atrial flutter without evidence of atrial fibrillation and were found to have intact isolation of the posterior wall. The authors conclude that “electrically isolating the posterior LA may thus prevent the initiation and perpetuation of AF”. This agrees with the findings in our patients to mean follow-up of 63-months.
Tamborero et al.13 randomised patients undergoing AF ablation to receive PV-encircling lesions and roof-line alone or supplemented with a floor line to complete PWI. A redo procedure was performed in 20% of their patients, and in 67% of initial PWI group, conduction across the roof-line was seen, suggesting that failure of the PWI model was in part due to reconnection. They found no advantage to PWI attempted through the addition of a floor line compared to PV-encircling lesions and roof-line only. Sanders et al. provide a rare example of AF recurrence in one of 27 patients following box isolation in whom the PWI remained intact at restudy.14 That index patient required additional “substrate modification” to restore sinus rhythm.
Kumagi et al.15 studied a purely paroxysmal group who, at mean follow-up of 13 months following catheter box-set ablation, showed a 95% rate of freedom from AF. Six of these patients required a redo procedure, three for recurrences of AF and the others from organised/focal arrhythmia. In all cases of recurrence, gaps in the lesions lines were seen. In none of these cases of recurrence was the PW isolated.
A recent resurgence in interest in the box set pattern has come about as a result of the development of hybrid AF ablation techniques, combining epicardial ablation using a variety of platforms with catheter-based endocardial ablation. This has resulted in reported outcomes in the region of 90%. These studies have the potential to offer some further insight into the electrical consequences of a single ring approach.5 To date, little has been published on the association between on-going AF prior to the second (catheter) stage of the hybrid pathway and the subsequent EP findings. As technology matures, this is a key opportunity to gaining a greater appreciation into the mechanisms of PsAF.
While it is clear that many features of the posterior LA wall mark it out as a key area of substrate for the propagation and perpetuation of AF, other areas of the atria may also be involved. Such sites as the superior vena cava, LA appendage base, interatrial septum and the anterior LA wall have all been implicated in AF propagation to a lesser extent.16 In addition, CFAEs,17 rotors and right atrial autonomic ganglia18 have also been shown to reside in the non-isolated area.
Oesophageal injury and fistulae formation is a rare but potentially fatal complication arising from ablation delivered onto the posterior wall.19 There is no instance of oesophageal complication in any of the reported literature on the box-set, but given the relatively low reported numbers, it is difficult to derive many conclusions from this given that the overall rate is estimated at 0.1%.20
Although the EP findings in patients with recurrent atrial arrhythmia following box isolation have previously been described, much less is known about long term invasive findings, especially in the group of patients with no recurrent arrhythmia. The bulk of the previous data comes from reports of patients undergoing transseptal procedures for organised atria arrhythmias. In the six patients free from AF in this present study, 4 had complete isolation of the PW, one had very delayed conduction through one gap near the right upper PV and one (who had experienced very little AF prior to surgery) had a non-isolated PW. The only patient who was AF free and was found to have a non-isolated PW was the only patient in the study with infrequent paroxysmal AF prior to surgery. This is maybe the reason for the clinical success despite the apparent electrophysiological failure. It may be that in such patients it is sufficient to modify the epicardial GP without necessarily achieving complete PWI for successful outcome.
Perhaps more interesting is our observation that in none of the patients with post-operative AF was the posterior wall isolation found to be intact. It can thus be hypothesised that the primary reason for failure to secure sinus rhythm is the failure of the technology used to deliver a complete transmural ablation ring, rather than the inappropriateness of the lesion pattern itself.
The aim of this study was to correlate the late clinical outcome with the electrical properties of the PW. The study achieved this endpoint. However, there are several limitations. Most significant is the low numbers included; a total of 17 patients of which only 6 had freedom from AF. This is in part due to the reluctance of patients with a clinically excellent outcome to undertake a further invasive procedure for no personal gain. The patient information provided was necessarily very comprehensive in describing potential adverse outcomes from what was on their part an entirely altruistic agreement to be studied. We observed a significant attrition rate during the waiting period between agreeing to enter the study and planned study date. All 31 patients who were contactable and eligible for inclusion in the sinus rhythm group were approached. Secondly, we would have hoped to gain an appreciation of the role of the ganglionic plexi (GP) in AF propagation post epicardial HIFU ablation. GP stimulation was excluded from the present study protocol. No initial ‘roadmap’ of their location had been made at time of surgery to guide which sites to address with high frequency stimulation. To attempt to stimulate all potential areas to would have prolonged the procedure beyond what was reasonable for these patients who had agreed to be studied under conscious sedation.
At long term follow-up, the decisive feature determining whether posterior wall isolation results in freedom from recurrence of atrial fibrillation appears to be either intact isolation of the area or extreme functional delay of conduction into and out of the box set.