Left Atrial Appendage Tachycardia Termination With A LARIAT Suture Ligation
Moustapha Atoui, Jayasree Pillarisetti, Sandia Iskandar, Matthew Earnest, Dhanunjaya Lakkireddy
Division of Cardiology, University of Kansas Hospital and Medical Center, Kansas City, KS.
Left atrial appendage (LAA) is a known trigger for left atrial tachycardia (AT). The use of LARIAT epicardial suture for termination of AT arising from LAA is not yet reported.
A 66-year-old female had a history of hypertension, diabetes, sick sinus syndrome, labile INR, and symptomatic persistent AF. She underwent radiofrequency ablation after failed cardioversion and multiple antiarrhythmics. She was in AT originating from LAA on activation map. Radiofrequency endocardial LAA isolation was performed. However, the AT recurred with increased burden and symptoms. Due to her multiple hospitalization for spontaneous bleeds and labile INR, a Lariat epicardial suture ligation of her LAA was performed. With application of the Lariat suture, electrical isolation was achieved and the AT terminated. She remained AT free at 18 months.
Our case is the first to illustrate the utility of LARIAT suture in electrical isolation of the LAA in addition to its mechanical exclusion.
Key Words : Atrial Tachycardia, LARIAT, Left Atrial Appendage.
The Left atrial appendage (LAA) is a known trigger for left atrial arrhythmia. While endocardial radiofrequency ablation of the LAA is well known to control atrial arrhythmias, the use of LARIAT epicardial suture for termination of AT arising from LAA is not yet reported.
A 66 year old female with recurrent symptomatic persistent atrial fibrillation (AF) and atrial tachycardia (AT) who failed cardioversion and multiple anti-arrhythmic medications including: propafenone, dofetilide, dronedarone and sotalol with several symptomatic breakthrough episodes.
Her past medical history included also: hypertension, diabetes mellitus, non critical coronary artery disease, obstructive sleep apnea, chronic obstructive pulmonary disease, colon cancer s/p colectomy, sick sinus syndrome s/p dual-chamber pacemaker, chronic anticoagulation with warfarin for AF and recurrent lower extremity deep venous thrombosis (DVT).
Physical examination and labs were unremarkable. After a lengthy discussion for the available options of her symptomatic arrhythmia she requested a radiofrequency ablation. Her CT scan showed normal pulmonary veins anatomy and a moderately enlarged left atrium with no thrombus. During her procedure and just after transseptal puncture, a thrombus was noted on the sheath inside the left atrium (despite INR=2.0 and 10,000 Units of Unfractionated IV Heparin bolus at transseptal puncture). The procedure was aborted and the patient was maintained on a higher INR goal (2.5-3.5).
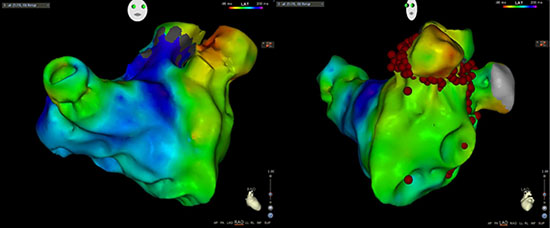
Unfortunately, with a supratherapeutic INR, a hematoma developed over her left elbow that had resolved later. She was rescheduled for the ablation procedure 3 months later. She was found in atrial tachycardia, cycle length (CL)= 470ms (127bpm) at the beginning of the procedure. This was tracked by her PPM (upper tracking rate 130bpm). Activation map of the tachycardia showed a reentrant tachycardia around the base and neck of the left atrial appendage (LAA) (Figure 1). She had extensive left atrial scar on voltage map. Isolation of the left atrial appendage with radiofrequency ablation successfully terminated the tachycardia and the patient converted to NSR. We continued the procedure with an isolation of her pulmonary veins and a cavo-tricuspid isthmus ablation for an induced right sided flutter.
Two weeks later, she presented with groin pain and hematoma. She was on enoxaparin subcutaneous injections for subtherapeutic INRs. CT scan showed a subacute right anterior thigh hematoma (5.9 x 2.7 cm) with no intra or retroperitoneal extension. Due to fluctuations in INRs a recommendation for new oral anticoagulants was made but the patient couldn’t afford it. The bleeding events unfortunately continued. With an INR of 3.5, she was admitted to an outsidehospital for hematomas in her psoas muscle and rectus abdominis
sheath.
Figure 1 RAO and LAO views of the AT Activation Map. Origin from the left atrial appendage. Ablation points of the LAA ablation were listed in the LAO view
She was in sinus rhythm at that time. She was restarted on
warfarin 2 weeks after the last bleeding event. Three months later the
patient was found with a recurrent atrial tachycardia with a similar
cycle length around 470ms. The burden increased and later become
persistent with atrial fibrillation reaching a 73% of the time on device
interrogation.
With recurrent atrial tachycardia that is most likely originating
from the left atrial appendage, multiple bleeding episodes from the
elbow, psoas, groin and rectus sheath; increased HAS-BLED score of
5 we discussed the treatment options with our patient and she agreed
for a LARIAT suture ligation for her left atrial appendage.
On the day of the procedure, and after intubation a negative transesophageal
echocardiogram for thrombus, the patient was noted in
atrial tachycardia. The CL was 470ms with ventricular pacing. The
Lariat device (SentreHEART, Redwood, California, USA) consists
of a compliant occlusion balloon catheter (EndoCATH), magnettipped
guidewires and a 12-F suture delivery device (LARIAT). The
procedure was done as described previously.1 To place the suture
across the LAA, a pericardial and transseptal accesses were obtained
separately. The endocardial magnet-tipped guidewire was advancedinto the apex of the LAA. The other magnet tipped guide wire was
advanced pericardially to the tip of the LAA to establish a stable
connection between the two magnet tips. A snare was then advanced
over the LAA and after confirmation of closure, a pre-tied suture for
LAA ligation was released and tightened (Figure 2). The tachycardia,
which was present throughout the procedure, terminated two minutes
after tightening the appendage and with further application of the
suture (Figure 3). She remained in normal sinus rhythm during her
uneventful post procedure stay.
During long term follow-up (18 months), her device interrogation
always showed great control of her atrial arrhythmia. The total AT/
AF burden decreased to 0.7%. She remains in AV sequential pacing
98% of the time with normal left ventricular function. Post her LAA
suture ligation she remained doing well off anticoagulation and was
kept only on Aspirin and Metoprolol.
Our case demonstrated the termination of left atrial appendage
reentrant tachycardia following appendage ligation with Lariat
suture delivery device. In our case, initial isolation of the appendage
was performed using radiofrequency ablation. However reconnection
occurred and electrical isolation was maintained with Lariat suture.
Although this case was mainly performed in the setting of recurrent bleeding episodes, however, this may open the potential use of Lariat
epicardial suture ligation for the treatment of atrial tachycardia
arising from the left atrial appendage. To our knowledge, this is the
first case report that demonstrates this phenomenon.
Figure 2 Left Atrial Appendage ligation with the Lariat Suture: A- The endocardial and epicardial magnet-tipped guidewires are connected and the Lariat device is placed around the neck of the appendage. B- Lariat device is tightened without releasing the suture, note that the balloon was deflated and the magnet-tipped guidewires are pulled back. C- After the satisfactory position, the Lariat suture was released, atriogram showing the final result
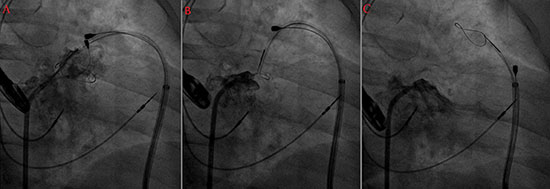
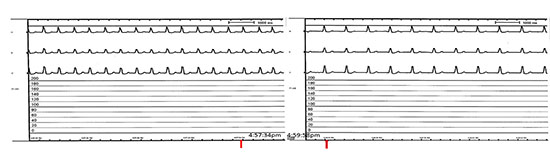
Figure 3 Electrical Isolation of the LAA with Lariat: termination of the AT was noted 2 minutes after tightening the appendage with Lariat Device/ Suture
LAA triggers are well known to be an important contributor for
initiation and maintenance of persistent atrial arrhythmias.2,3 If
the LAA was found to be a trigger (like in this case), endocardial
isolation of the left atrial appendage reduces the risk of AF/
AT.2 However, endocardial isolation of the LAA is extremely
challenging as it carry a significant risk of perforation, in addition to
electromechanical dissociation which may subsequently contribute to
thombus formation.2 In our patient with recurrent bleeding episodes,
the choice of a LAA ligation with Lariat suture was optimal as it
provided both mechanical as well as electrical isolation of the LAA.
The later most likely decreased the risk of stroke in this patient with
persistent arrhythmia.
A prior case report demonstrated the termination of the atrial
tachycardia immediately after the placement of the AtriClip device.4
Similarly in preclinical studies epicardial LAA exclusion has been
shown to result in LAA electrical isolation.5 In a recent study
performed at our center, we demonstrated that 90% of patients had
a reduction in the LAA voltage with lariat suture ligation. We were
also able to demonstrate the lack of left atrial capture during bipolar
pacing from the occluded LAA in 90% of the patients tested.6
Although various other delivery devices like the watchman device and
the amplatzer cardiac plug are good mechanical closure alternatives
and help to prevent risk of thromboembolism, however electrical
isolation cannot be achieved. Arrhythmia control was extremely
helpful in our patient and she was able to remain asymptomatic while
off antiarrhythmic and anticoagulation. This was recently proven in
our prospective observational study, LAALA-AF, which involved
138 patients with persistent AF. A higher freedom of AF at 1year
off antiarrhythmic was noted in the Lariat group (65% VS 39%,
p=0.002) in addition to less need for a repeat ablation for recurrence
(16% VS 33%, p=0.018).7
Procedure wise, Lariat ligation suture was shown to have a high
success rate (96%, 89 patients) and limited adverse events rate of 3.3%
(bleeding).8 This rate was even lower in a large survey conducted in
11 US sites that involved 441 patients. The bleeding rates decreased
from 3.3% (2% need for transfusion and 1.3% need for open heart
surgery) to none in the remaining 231 patients and was referred to
the switch from an 18-gauge Pajunk needle to micropuncture for
the pericardial access.9 The use of micropuncture needle (performed
in this case) was further recommended in a multi-center study
including our center. With its use, a decreased incidence of major
complications and the need for surgical repair was noted.10
In conclusion, our case is the first reported which demonstrates
termination of a LAA reentrant tachycardia with a Lariat suture. It
may be important to consider the electrical isolation as additional
benefit to patients who are selected for appendage occlusion.