Pulmonary Vein Isolation with the Multipolar nMARQâą Ablation Catheter: Efficacy And Safety In Acute And Long-Term Follow Up
Siebermair Johannes1,3, Michelle Silver2, Wakili Reza11,3,4
1Department of Medicine I, University Hospital Munich, Ludwig Maximilians University, Munich, Germany.2Comprehensive Arrhythmia Research & Management (CARMA) Center, University of Utah School of Medicine, Salt Lake City, Utah, USA.3Deutsches Zentrum fĂŒr Herz-Kreislauferkrankungen (DZHK), partner site Munich Heart Alliance, Munich, Germany.4Department of Cardiology and Vascular Medicine, West-German Heart and Vascular Center Essen, University of Essen Medical School, University Duisburg-Essen, Essen, Germany.
Pulmonary vein isolation (PVI) is an established therapy for atrial fibrillation (AF). One challenge in the catheter-based treatment of this arrhythmia is to develop an effective and safe ablation approach to achieve durable and consistent lesions around the PVs. The multipolar irrigated radiofrequency (RF) ablation catheter nMARQTM was designed as a single-shot device with the aim to achieve these goals. This article reviews the current literature with respect to acute- and long- term success rates after PVI with this circular mapping and ablation device. Furthermore, since this device recently became discredited to potential lethal complications, we will also focus on the data available on safety issues with this ablation system.
Key Words : nMARQ, circular ablation catheter, atrial fibrillation, pulmonary vein isolation.
Correspondence to: Reza Wakili, MD; MarchioninistraĂe 15, 81377 Muenchen, Germany; Tel.: +49 89 7095 3036; Fax: +49 89 7095 8767; E-mail: Reza.Wakili@med.uni-muenchen.deâ
Pulmonary vein isolation (PVI) is an established method for the treatment of atrial fibrillation (AF). In 1998, Michel HaĂŻssaguerre demonstrated that the pulmonary veins (PV) were an important anatomical structure harboring triggers for the initiation of AF [1]. Thus, the primary endpoint for interventional treatment of AF by ablation is circumferential electrical isolation of the PVs [2]. However, as this procedure is challenging and still time-consuming even for experienced operators, there is a need for workflow optimization, e.g. by novel ablation devices. In this context, so-called âsingle-shotâ devices have been introduced in order to enable a quick and durable PV isolation, thereby increasing efficacy and safety of PVI procedures. Single-shot devices were developed as a tool aiming to provide circular transmural lesions by simultaneously mapping and ablating at multiple sites around the antra of the PVs via a single-transseptal access point [3].
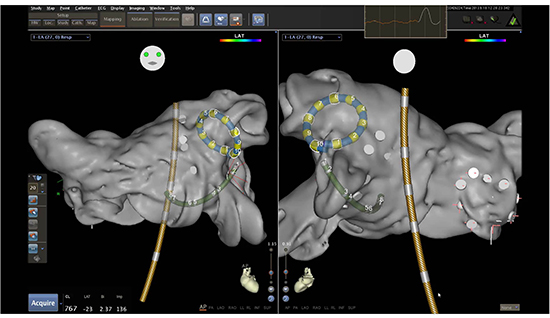
In 2011, a steerable multi-electrode catheter (8.4 F) with a deflectable tip (nMARQTM, Biosense Webster, Inc., Diamond Bar, Ca, USA) was introduced. The nMARQTM catheter consists of ten irrigated electrodes, and is capable of full integration into the CARTOÂź electroanatomic mapping system [4] [Figure 1]. Energy delivery duration is set between 30 to 60 seconds, and radiofrequency (RF) ablation can be individually performed over each combination of the 10 electrodes in unipolar mode (maximum 25 W) or bipolar mode between two electrodes (maximum 15 W) [5].
Figure 1. illustrates the position of the circular nMARQTM catheter in the left atrium, recording the electroanatomical map in CartoÂź 3. Yellow-grey: temperature probe; green: RV catheter for phrenic nerve stimulation during ablation of right-sided PVs. Figure courtesy of Dr. Wakili
Early studies suggested the nMARQTM to be an effective and safe tool for PVI [4], [6]-[8] with one multicenter study confirming a high success rate after nMARQTM procedures [3]. However, the device also presented with some safety concerns arising from some severe complications, questioning the safety of this novel device [9]-[11], and ultimately leading to the interim recall of the 2nd generation nMARQTM catheter [12]. Therefore, we conducted this review of the current literature with respect to mid- and long-term efficacy as well as safety of the nMARQTM ablation device.
The electronic databases PubMed and Google Scholar were used to identify potential articles including prospective and retrospective studies, case reports, registries, editorials, and review articles. Search terms included âatrial fibrillation (AF),â âpulmonary vein isolation (PVI),â âcircular ablation catheter,â âmultipolar ablation catheter,â âsingle shot device,â and ânMARQTM.â Data from 81 identified articles were reviewed carefully for information regarding ablation with the nMARQTM device. We summarized the data according to the available information on clinical outcome (n=11 studies), procedural parameters (n=14 studies) and safety outcome (n=16 studies).
Since the release of the first generation of this catheter in 2011, outcomes following nMARQTM ablation from more than 1400 patients have been reported [3], [4], [6], [7], [9], [13]-[18]. Specifically, our search found 11 published studies, with follow-up (FU) data exceeding 3 months post PVI. The outcome of interest in these studies was generally defined as recurrence of AF or the combination of AF with atrial flutter and atrial tachycardia following a 90-day blanking period after PVI. Results from one multicenter study, and 10 single-center studies reported overall mid-term success rates ranging from 52% to 80.9%. Success rates varied depending on AF type, FU duration, and the concomitant use of antiarrhythmic drugs (AAD) [Table 1].
Table 1. Clinical outcomes of patients investigated in available literature. FU denotes follow-up; AAD antiarrhythmic drugs
|
Study
|
Patient number
|
Paroxysmal AF (n)
|
Paroxysmal AF (%)
|
FU (months)
|
Recurrence rate
|
AAD
|
|
Vurma, 2016
|
327
|
228
|
69.7
|
6±5
|
25% paroxysmal
|
OFF AAD
|
|
Â
|
 |
 |
 |
 |
48% persistent
|
Â
|
|
Wakili, 2016
|
29
|
29
|
100
|
12.4±9.3
|
28%
|
OFF AAD
|
|
Rodriguez-Entem, 2016
|
35
|
35
|
100
|
16.8±2.8
|
22.8%
|
ON AAD
|
|
Laish-Farkash, 2016
|
82
|
62
|
75.6
|
12
|
19.3%
|
ON AAD
|
|
Burri, 2016
|
50
|
50
|
100
|
15±4
|
54%
|
OFF AAD after blanking
|
|
Stabile, 2015
|
180
|
140
|
78
|
13.9±8.2
|
27% paroxysmal
|
OFF AAD
|
|
Â
|
 |
 |
 |
 |
30% persistent
|
at discretion of physician
|
|
Mahida, 2015
|
374
|
263
|
70.3
|
12
|
35% paroxysmal
|
20% ON AAD
|
|
Â
|
 |
 |
 |
 |
35% persistent
|
30% ON AAD
|
|
Deneke, 2015
|
145
|
77
|
53.1
|
12
|
31% paroxysmal
|
Â
|
|
Â
|
 |
 |
 |
 |
38% persistent
|
Â
|
|
Zellerhoff, 2014
|
39
|
39
|
100
|
4.7±2.5
|
34%
|
OFF AAD after blanking
|
|
Shin, 2014
|
25
|
25
|
100
|
4.1±1.6
|
19.1
|
OFF AAD
|
|
Scaglione, 2014
|
25
|
25
|
100
|
6
|
32%
|
OFF AAD
|
For those patients that underwent ablation of paroxysmal AF, recurrence rates were between 22.8% and 35% [3], [16], and are comparable to those obtained by conventional RF, Cryoballoon, or different circumferential RF ablation catheters (PVAC) after one year (between 30.1 - 35.9%) [19]-[21]. The very low recurrence rate of 22.8% reported by Rodriguez et al. may be in part attributable to the fact that all patients were administered AAD during the blanking period [16]. Burri et al. reported recurrence rates of 54% over 15 ± 4 months which were considerably higher than other published studies [13]. The authors suggested that in addition to the slightly longer FU duration compared to other studies, reduced power output (max. 15 watt unipolar), the restricted RF delivery, and the waiving on a circular mapping catheter to confirm PVI, could be causative for worse outcomes in their study [7], [18] (see chapter âacute efficacyâ).
Data on success rates after nMARQTM ablation in persistent AF are scarce. The five published studies following patients with persistent AF reported recurrence rates ranging from 30% to 48% [3], [9], [14], [15], [17]. The clinical use of the nMARQTM device has been limited so far in patients with persistent AF. Prior expert consensus documents from the HRS/EHRA/ECAS suggested that for patients with persistent AF âoperators should consider more extensive ablation based on linear lesions or complex fractionated electrogramsâ [22], for which the nMARQTM catheter is not intended. Notably, since Verma et al. reported that ablation strategies beyond conventional PVI did not translate into additional clinical benefit in persistent AF in the STAR-AF-II trial, the use of the single-shot devices, incl. the nMARQTM catheter, re-gain attention for a PVI only treatment in patients with persistent AF [23].
Acute efficacy of nMARQTM guided ablation
Acute durable PVI (acute efficacy) with the nMARQTM device ranged from 83% to 100% of treated patients, with acute efficacy in 95.7% to 100% of targeted veins [4], [18], [24] (see [Table 2]). Wakili et al. reported that 5 of 116 PVs (4.3%) could not successfully be isolated with the nMARQTM catheter [18]. Zellerhoff et al. failed to acutely isolate three PVs (2x RSPV, 1x RIPV), Rodriguez-Entem, 2 PVs (1 RIPV and 1 LIPV), and Scaglione, 1 LSPV [6], [7], [16]. Indicated reasons for isolation failure comprised of difficulties in achieving a transmural lesion at the ridge, significant temperature rise in esophagus, catheter geometry, and limited device maneuverability [7], [18]. According to their single-center experience, Deneke et al. reported that through the routine use of a steerable sheath for catheter access into LA, when appropriate contact force in the LAA ridge region is achieved, all different anatomies of PVs should be treatable by the nMARQTM device [8], [14]. Inconsistent with results from PVI with single-tip catheters and circular mapping catheters (CMC), most of the reported studies did not routinely perform exit block testing to confirm PVI. This was due to challenging intubation of small PVs with the nMARQTM catheter [18].
Table 2. summarizes acute success rates and procedural results with the nMARQTM ablation device. * highlights studies with PVI confirmation with additional circular mapping catheter
|
Study
|
no. of pts.
|
acute PVI success, n
|
isolated PVs
|
Targeted veins (%)
|
total procedure time (min)
|
Fluoroscopy time (min)
|
RF time (min)
|
Anesthesia
Sedation
|
|
Vurma, 2016
|
327
|
 |
 |
 |
69±22 paroxysmal
|
14.8±6.6 paroxysmal
|
18.9±6.4 paroxysmal
|
general anesthesia
|
|
Â
|
 |
 |
 |
 |
75±23 persistent
|
16.8±6.3 persistent
|
22.1±6.1 persistent
|
Â
|
|
Rodriguez-Entem, 2016
|
35
|
33 (94.3%)
|
138/140
|
98.6
|
 |
 |
 |
mainly conscious sedation
|
|
Laish-Farkash, 2016
|
82
|
78 (95%)
|
 |
 |
81±18
|
30±8.5
|
11±4
|
conscious sedation
|
|
Burri, 2016
|
50
|
50 (100%)
|
 |
 |
100±25
|
22±8
|
 |
conscious sedation
|
|
Stabile, 2015
|
180
|
176 (97.8%)
|
 |
98
|
113±53
|
13.1±8.4
|
12.5±5.1
|
not specified
|
|
Mahida, 2015
|
374
|
 |
1468/1474
|
99.6
|
114±42
|
24.4±14
|
13.5±6.4
|
not specified
|
|
Rillig, 2015
|
21
|
20 (95.2%)
|
87/88
|
98.9
|
223±53
|
35.5
|
 |
conscious sedation
|
|
Deneke, 2015
|
145
|
 |
556/559
|
99.5
|
115
|
17.25
|
18.5
|
not specified
|
|
Zellerhoff, 2014
|
39
|
37 (94.9%)
|
151/154
|
98.1
|
86±29
|
22.2±6.5
|
10±4.6
|
conscious sedation
|
|
Shin, 2014
|
25
|
25 (100%)
|
97/97
|
100
|
110±31
|
23±9
|
15±6
|
conscious sedation
|
|
Scaglione, 2014 *
|
25
|
24 (96%)
|
100/102
|
98
|
131±49
|
1.8±2
|
14.9±3.7
|
conscious sedation
|
|
Wakili, 2016*
|
29
|
24 (83%)
|
111/116
|
95.7
|
132.1±36.6
|
30.5±11.7
|
21±9
|
conscious sedation
|
|
Kiss, 2014
|
14
|
98%
|
 |
 |
108±25
|
21.1±7.8
|
7.7±3.4
|
conscious sedation
|
|
Rosso, 2014*
|
10
|
10 (100%)
|
Â
|
100
|
109.3±38.4
|
31.3±11.2
|
Â
|
both
|
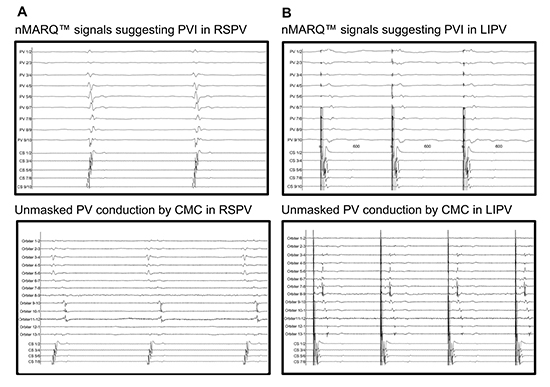
With respect to acute success rates of PVI, these results are comparable to those obtained by conventional RF energy [25], PVAC [21], [26] and Cryoballoon ablations [25], [27]. However, most of these studies used the nMARQTM as the intended âsingle-shotâ device, without confirming the PV isolation with a standard CMC. Scaglione and Rosso et al. reported on an overall inconsistency between CMC and nMARQTM signals in 22 of 102 PVs (22%) to 12 of 39 PVs (30%). Additionally, Rosso observed good consistency prior to PVI, but poor concordance after PVI. In all cases these variations led to further RF delivery [7], [24]. Wakili et al. reported on a discrepancy rate of 35% in their study [18] [Figure 2]. Scaglione et al. speculated that persistent PV potentials on the CMC after extinction on nMARQTM suggest persistence of electrical conduction from the PV to the atrium. They suggested that the difference in inter-electrode spacing between CMC and nMARQTM, or the more proximal position of the nMARQTM in the PVs, are causative for significant signal divergence [7], [28], [29]. In order to avoid false-positive PVI results which may impair the outcome of the procedure, Wakili et al. strongly recommended a dual transseptal approach with simultaneous PV potential recordings [18].
Figure 2. Insufficient signal accuracy of the nMARQTM catheter [18]. The illustration shows intracardiac recordings of consecutive PV mappings by the nMARQTM catheter and by a CMC of the same vein after ablation; (A) RSPV mapping with nMARQTM suggests absence of PV conduction (upper panel) and subsequent CMC mapping shows persisting conduction in RSPV at electrodes 9â12 (lower panel); (B) Differential pacing: LIPV mapping with nMARQTM (upper panel) suggests absence of PV conduction; subsequent CMC mapping unmasks persistent conduction in LIPV on CMC electrodes 3â13 (lower panel).
Deneke et al. suggested that there may be procedure-related factors influencing the success rates following ablation with the nMARQTM device. In particular, Deneke et al. reported that overall success rates were positively associated with higher maximum energy delivery rates at the posterior wall (25 watt vs. 20 watt) [14]. However, these higher energy delivery rates were likely associated with a higher risk for esophageal thermal damage [5].
The development of the nMARQTM as a single-shot device was driven by the intention to shorten and simplify PVI procedures, increase safety, and reduce radiation dose, all while producing equal (or better) success rates of other ablation devices. Pooled results for periprocedural data are depicted in [Table 2].
Total procedure times ranged from 72 ± 6.5 minutes [9] to 223 ± 53 minutes [5]. Total procedure time in the latter study is likely highest due to four cavotricuspid isthmus ablations, and one ablation of roof dependent LA tachycardia which was performed during the course of PVI. Summarized, total procedure times reported from the nMARQTM device compared well with procedure times obtained from other PVI ablation modalities (Cryoballoon: 136 to 371 min [20], [30], [31]; PVAC: 121 to 137.1 min [32], [33]; RF 140.9 to 165 min [19], [25]). Multiple groups suggested after a learning period a mean reduction in overall procedure time of 19.1% to 62.1% [4], [14], [24]. However, Wakili et al. failed to show a significant nMARQTM ablation learning curve with respect to overall procedure time [18].
Mean fluoroscopy times varied over a broad range, from 1.8 minutes [7] to 35.5 minutes [5]. In the latter study, the prolonged fluoroscopy times may be explained by additional CMC use in order to confirm complete PVI. Ablation with the nMARQTM reveals comparable fluoroscopy times as indicated in literature for other ablation devices (Cryoballoon: 21 to 40 min [19], [30], [31], [34]; PVAC 21 to 33 min [21], [32]; single tip 16.6 to 24 min [19], [25]. A suggest learning curve shows a reduction of 51.5% to 64.5% of total fluoroscopy time [4], [24].
With respect to total RF time, as the number of active electrodes during ablation can individually be varied, the comparison to different PVI modalities is challenging [9]. When reporting on RF duration, the majority of studies reported the total RF duration, without indication of the number of active electrodes. This hampers the direct comparison of RF times to other one shot devices or single-tip catheter approaches. However, total nMARQTM RF times (7.7 to 18.5 min [9], [35]) are slightly longer compared to reported RF durations with conventional single tip catheters (33 min [25]; 21 min [18]). Only three studies used an additional CMC to confirm complete PVI [7], [18], [24]. Wakili et al. reported that the use of an additional CMC to confirm PVI was associated with longer RF durations, and with the identification of 19 of 29 PVs (65.5%) with persisting atrio-PV conduction after nMARQTM ablation (21.0 ± 9.0 vs. 17.6 ± 6.5 min) [18]. Data on analyses of RF times per individual vein is scarce. The available literature provides evidence that RF times needed for PVI are significantly longer in the superior PV compared to RF times needed in the inferior PVs [3], [7], [16]. All but one study indicates that mean RF times with the nMARQ device are longest in the LSPV (191.6 ± 41.9 sec).
As the nMARQTM catheter has shown to be associated with comparable outcomes to currently available ablation technologies, in respect to recurrence post ablation, a specific focus is placed on safety issues. In general, AF ablation is associated with a incidence of acute complications ranging from <1% to 6% [36].
Esophageal thermal damage
Due to the specific design of circular ablation devices and therefore high energy delivery at the posterior wall, esophageal lesions are of major concern [Table 3]. Esophageal thermal damage (ETD) is considered a precursor of fistulas, even though the causal relation between fistulas and thermal esophageal lesions is largely unclear [14]. Following PVI with a single-tip, a high incidence of thermal lesions have been reported (thermal esophageal damage (11%) and gastroparesis (17%)) [37]. Deneke et al. assessed 136 out of 145 patients with endoscopy after nMARQTM ablation, and report on 7 ulcerous and 22 erythematous lesions after PVI with the nMARQTM [14].
ETD resulting in fistulas can lead to fatal complications. The indicated mortality in literature after development of atrio-esophageal fistula (AEF) is 71% [38]. An overall incidence of 3 of 1417 patients (0.21%) that developed AEF has been derived from the published nMARQTM studies. Of those reported cases of AEF, Vurma et al. reported fatal outcomes following development of AEF in 2 of 327 consecutive patients (0.6%) following ablation with the nMARQTM device. This report led to an immediate recall of the nMARQTM catheter in its 2nd generation [9], [12]. Deneke and Mahida et al. each reported cases of delayed occurrence of AEF, the latter reporting on a delay of 4.5 weeks between PVI procedure and occurrence of first symptoms [3], [11].
Various safety precautions have therefore been suggested in order to avoid thermal esophageal damage. According to initial experience, the use of a thermal probe has been suggested in order to reduce the incidence of thermal damage during AF ablation at the posterior wall [39], [40]. Disagreement remains on the esophageal cut-off temperature during RF delivery, ranging from 39 degrees [41] to 41 degrees Celsius [5], [42].
Considering the recent literature on nMARQTM ablations, only one study [43] suggested a benefit of using a temperature probe during multipolar RF ablation [14], [35], [41], [44]. Consistent with other reports concerning RF ablation, Deneke et al. suggested an increased risk for ETD in patients with thermal probes during RF ablation (21% vs. 0%, p<0.001) [5], [8], [14], [18]. They speculate a possible âantennaâ effect of the thermal probe intensifying local energy with heating at the esophageal region, or a stiffening of the esophagus itself avoiding the esophagus to sidestep during catheter pressure [5], [14], [39], [45]. However, in cases of large esophageal diameter, the probe is not able to cover the entire esophageal region (as shown by barium sulfate ingestion), and therefore may lead to an underestimation of the local temperature. This underestimation of temperature may result in a higher risk for esophageal thermal lesion [41]. According to those presented data, the use of thermal probes should therefore be avoided.
Other precautions suggested for ETD prevention comprise a reduction of the maximum power (20 watt [14]), and even lower temperatures when bipolar ablation is performed [41]. Limitation of RF time at the posterior wall is also recommend for ETD prevention [5]. The two cases of AEF reported by Vurma et al. occurred following ablation with a max. temp of 16 to 18 watts (30 sec max. duration for vast of energy deliveries) [9]. It must be mentioned that the report of maximum delivered RF energy is often misleading. In order to avoid ETD, most operators only decrease RF power at the posterior wall.
Finally, the use of general anesthesia has been reported to serve as a risk factor for ETD [46]. Most of the patients undergoing ablation under general anesthesia also had esophageal temperature probes during the procedure. Therefore, the influence of general anesthesia as a risk factor for thermal lesions remains unclear, and needs to be critically questioned.
Thromboembolic complications
Thromboembolic complications are generally considered a major concern with the nMARQTM device, which is based on former negative experience with the circular single-shot ablation PVAC device [47], [48]. Reviewing the current literature on nMARQTM ablations, no stroke or transient ischemic attack (TIA) were reported. However, silent cerebral lesions (SCL), which likely represent small thromboembolic infarctions, have been reported in literature. Varying based on the ablation technology used, SCL were reported in up to 40% of patients after RF ablations [14], [47], [49], [50]. Since embolic-lowering maneuvers have been introduced into clinical practice, the use of the nMARQTM device remains associated with the highest reported incidence of asymptomatic thromboembolic complications [6], [8], [51]. The clinical significance of these SCL is unclear. However, an association between SCL and neuropsychological changes, especially of verbal memory, has been suggested [52], yet other studies have failed to show an association [53], [54].
Out of 16 reported studies on complication rates, six studies performed cerebral imaging (CT\MRI) after PVI to rule out SCL [4], [6], [8], [14], [16], [53]. Two groups found SCL following PVI with the nMARQTM device, ranging from 1 in 19 patients (5%) [53] to 14 in 43 (33%) [55]. However, none presented with any obvious neurological symptoms. The high percentage of 38% post-ablation SCL, as indicated by Deneke et al.[55], might overestimate the real percentage as Sugihara et al. found a high incidence of preexisting SCL before PVI [53]. This high prevalence of pre-existing lesions (12.3-92%) might represent a condition of inappropriate anticoagulation before PVI [50], [51], [54]-[57]. In this context, studies have indicated that the maintenance of preexisting anticoagulation, compared to discontinuation and bridging with heparin, contributed to a reduction of periprocedural cerebral events [58], [59]. In general, different anticoagulation regimens make the comparison of studies dealing with microbubbles during ablation difficult [35]. Kiss et al. demonstrated that nMARQTM ablation was associated with a high incidence of microbubbles. This bubble formation seems to be higher than when compared to ablation with new-generation PVAC devices, or cryoballoon ablation [35]. The assessment of the intensity of micro emboli generation during ablation procedures is measured in the middle cerebral artery by transcranial Doppler [35]. However, this technique of measuring microbubbles by ultrasonic techniques has not been consistently validated with respect to the clinical significance. It remains completely unclear as to whether these microbubbles represent solid particles or gas and how they translate into a manifest clinical finding.
With respect to conditions predisposing to thrombi formation, the specific design of the circular nMARQTM catheter with 10 irrigated electrodes is suspected to be causative for this phenomenon. Csanadi et al. speculated that the high volume flow of irrigation saline solution (6-7ml/ electrode, resulting in 60-70ml/min) can result in bubble formation and subsequent microembolism [60]. Further, charring on the electrodes is thought to be another major source of SCL, arising from former PVAC experience [5]. Shin reported the identification of charring on 3 of 15 cases (20%) with the nMARQTM catheter [4]. Charring was found primarily between electrode 1 and 10. This location is where electrodes are delivering RF energy in close proximity, and is likely the source of a bipolar short circuit resulting in tissue and blood heating [51]. Therefore, Shin et al. recommend RF delivery only with sufficient distance between electrode 1 and 10 on fluoroscopy, 3D visualization, without indication of proximity by artifacts on the corresponding EGMs [4].
Despite existing data from animal studies investigating the PVAC device, there is still discrepancy as to whether the use of unipolar over bipolar RF energy per se could reduce the incidence of microembolism [61]. Nevertheless, in order to reduce the incidence of SCL, abandonment of a bipolar RF energy use is recommended in general now. As catheter manipulations are thought to be a source of microbubble formation, the following precautions should be considered [54], [59]: at least half the calculated bolus dosage of heparin should be given before transseptal passage, continuous flushing of the long LA sheath, and whenever possible, retraction of the sheaths in RA. Additionally, a catheter change over the long LA sheath should be avoided [35]. This however questions the intention of these âsingle-shotâ devices, because in addition to the PVI, an additional CMC may be required [7], [18], [24]. Further, the administration of a proton pump inhibitor should be considered for at least 6 weeks following ablation in order to prevent progression of esophageal thermal damage to ulceration [5], [8].
Other severe complications
Other severe complications including pericardial effusion/tamponade and phrenic nerve palsy (PNP) were reported in 7 out of 16 cited studies [5], [6], [10], [13]-[16], [18]. Pericardial effusion/tamponade was reported in 6 out of 1417 (0.4%) patients, and PNP in 4 of 1417 patients (0.3%). However, the prevalence of PNP following nMARQTM ablation is lower than in the literature for overall PVI procedures, with PNP rates ranging from 0.48% to 11% [62]-[64]. Although injury of the phrenic nerve is reported following various ablation techniques, it has been suggested to be more likely with the Cryoballoon [20], [65]. The exact mechanism of the high rate of PNP after circular PV ablation with the Cryoballoon remains unclear, especially with regard to the lower percentage of PNP after nMARQTM of overall 0.3% [10], [13], [66]. This may be explained in part by a more antral ablation with nMARQTM catheter compared to Cryoballoon (diameter 20 to 35mm vs. 23 or 28mm) [6]. With respect to the underlying mechanism, experimental data suggested a Wallerian degeneration (axonal damage by coagulation), or an injury of the right pericardiophrenic artery, both with the potential for recovery [62], [67], [68].
In order to avoid PNP during nMARQTM ablation, Arroja et al. suggested a further power limitation of 12 to 15 watts, phrenic nerve stimulation on each electrode of the nMARQTM catheter, and continuous phrenic nerve stimulation during RF application [10]. Additionally, Roka et al. reported on a novel technique to prevent PNP, by identifying the overlapping region between right and left atrium. RF applications proximal to this line are suggested to be safe with respect to PNP [62]. In order to rule out pulmonary vein stenosis following PVI, imaging modalities were reported on in five studies [Table 3], and no significant stenosis was mentioned.
Table 3. An overview of published literature on procedure-related complications with the nMARQTM system. PE denotes pericardial effusion/tamponade; PNP phrenic nerve palsy; AEF atrio-esophageal fistula; SCE silent cerebral lesion; TP temperature probe; EGD esophago-gastro duodenoscopy; ETD esophageal thermal damage; LA left atrium; PVS pulmonary vein stenosis; PN phrenic nerve; RF radio-frequency; TIA transient ischemic attack
|
Study
|
Pt. No.
|
PE
|
Access
site
|
ECG
alteration
|
PNP
|
Stroke\ TIA
|
AEF
|
ETD
|
SCE
|
death
|
PVS
|
MRI\ CT LA
|
TP
|
EGD
|
PN test
|
RF
|
|
Vurma, 2016
|
327
|
0
|
13
|
2
|
0
|
0
|
2
|
 |
 |
2
|
0
|
No
|
no
|
no
|
 |
16-18 W
|
|
Rodriguez-Entem, 2016
|
35
|
1
|
 |
 |
0
|
0
|
0
|
 |
 |
0
|
0
|
yes (n=19)
|
 |
no
|
yes
|
20-25 W uni
|
|
Laish-Farkash, 2016
|
82
|
1
|
4
|
3
|
 |
0
|
0
|
 |
 |
0
|
 |
no
|
 |
no
|
 |
15-20 W uni
|
|
Burri, 2016
|
50
|
2
|
 |
0
|
1
|
0
|
0
|
 |
 |
0
|
 |
no
|
no
|
no
|
yes
|
15 W uni
|
|
Knecht, 2016
|
40
|
0
|
0
|
0
|
 |
0
|
0
|
 |
 |
0
|
 |
no
|
yes
|
no
|
 |
15-20 W uni
|
|
Stabile, 2015
|
180
|
0
|
 |
0
|
0
|
0
|
0
|
 |
 |
0
|
 |
no
|
no
|
no
|
yes
|
20-25 W uni
|
|
Mahida, 2015
|
374
|
0
|
 |
0
|
0
|
0
|
1
|
 |
 |
2
|
0
|
0
|
no
|
no
|
 |
25 W uni- and bipolar
|
|
Rillig, 2015
|
21
|
0
|
 |
0
|
1
|
0
|
0
|
4
|
 |
0
|
 |
0
|
yes
|
yes
|
 |
10-20 W uni- and bipolar
|
|
Deneke, 2015
|
145
|
1
|
0
|
0
|
1
|
0
|
1
|
29/Â 136
|
26/Â 115
|
1
|
0
|
yes
|
103/ 145
|
yes
|
yes
|
20-25 W uni
|
|
Di Monaco, 2015
|
30
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
 |
0
|
 |
no
|
yes
|
yes
|
yes
|
15-18 W uni- and bipolar
|
|
Arroja, 2015
|
1
|
0
|
0
|
0
|
1
|
0
|
0
|
0
|
0
|
0
|
0
|
 |
 |
 |
no
|
15 W
|
|
Zellerhoff, 2014
|
39
|
1
|
 |
 |
 |
 |
 |
 |
 |
0
|
0
|
yes
|
no
|
no
|
no
|
25 W uni
|
|
Shin, 2014
|
25
|
0
|
0
|
 |
0
|
0
|
0
|
 |
 |
0
|
0
|
yes
|
no
|
no
|
yes
|
20-25 W uni
|
|
Scaglione, 2014
|
25
|
0
|
3
|
0
|
0
|
0
|
0
|
 |
0
|
0
|
 |
0
|
no
|
no
|
yes
|
18-20 W uni
|
|
Kiss, 2014
|
14
|
0
|
 |
 |
 |
 |
 |
 |
 |
0
|
 |
 |
 |
 |
 |
20 Watt uni
|
|
Wakili, 2014
|
29
|
0
|
1
|
0
|
1
|
0
|
0
|
1
|
Â
|
0
|
0
|
0
|
yes
|
yes
|
yes
|
18-20 W uni
|
The nMARQTM catheter was developed in order to enable fast, durable, and safe PVI by using a single-transseptal approach. As presented, the literature reveals comparable acute and long-term clinical outcomes after AF ablation to single-tip and different other circular ablation catheters. With respect to procedural parameters, current studies failed to provide an evidence for reduction of total RF with the nMARQTM device (see [Table 2]). However, these studies comprised initial experience, scientific evaluation with a learning curve and small patient cohorts in a majority of studies.
Although intended to function as a single-shot device, some issues were presented concerning the catheterâs procedural performance. For example, when using the device via the intended single-transseptal approach without CMC confirmation, this results in insufficient PVI, and may be impairing ablation success rates. In order to perform this additional CMC assessment of complete PVI, it must be advanced in to LA by catheter change, or dual-transseptal approach. These approaches increase complexity and prolong procedure and fluoroscopy times. Further, catheter change in the LA is suggested to be associated with microembolism. Therefore, the intended single-shot character of this device requires investigation in larger prospective trials with strict intention-to-treat designs.
Still despite establishing safety precautions, a high rate of esophageal thermal damage and atrio-esophageal fistulas were reported with the nMARQTM device, especially with the 2nd generation device. This is of major concern as these injuries often result in fatal outcomes. Following the re-launch of a new nMARQTM generation, further investigation into the safety of this device with respect to esophageal thermal damage is absolutely essential prior evaluating the clinical efficacy. In general, based on the early and limited experience with few severe complications associated with the nMARQTM device, a close FU of patients after PVI with all circular mapping devices should be aimed for.
In sum, following the idea of an easy-to-use and efficient ablation tool enabling fast and complete PVI, the nMARQTM catheter has proven feasibility, but still needs further evaluation in order to establish a reliable safety profile before aiming for superiority with respect to procedural and clinical variables in larger trials.
Dres. med. Siebermair and Wakili were supported by the German Centre for Cardiovascular Research (DZHK). Dr. Siebermair received by the FöFoLe-Programme of the University of Munich, Munich, Germany. Dr. Wakili received funding from the European Unionâs Horizon 2020 research and innovation programme under grant agreement No 633193 (CATCH ME).