Elevated Left Atrial Volume Index Predicts Incident Atrial Fibrillation After Typical Right Atrial Flutter Ablation
Justyna Rzucidlo, Priya Panday, Marissa Lombardo, Eric H. Shulman, David S. Park, Scott A. Bernstein, Lior Jankelson, Douglas Holmes, Anthony Aizer, Larry A. Chinitz, Chirag R. Barbhaiya
Leon H. Charney Division of Cardiology. New York University Langone Health. New York, NY, USA.
Incident atrial fibrillation (AF) is common after cavotricuspid isthmus (CTI) dependent atrial flutter (AFL) ablation. Risk factors for the development of AF post ablation are not well understood. The purpose of this study was to identify patients undergoing CTI ablation for AFL most likely to develop AF.
Retrospective chart review identified 114 consecutive patients without a history of AF or prior cardiac surgery who underwent typical CTI dependent AFL ablation between December 2013 to November 2018, who also had a complete preoperative transthoracic echocardiogram, and at least 1 year of follow-up at our medical center. We evaluated baseline characteristics, electrophysiology study (EPS) data and echocardiographic data for incidence of AF within 3 years.
Incident AF was identified in 46 patients (40%) during 600 + 405 days follow-up. Left atrial volume index (LAVI) was significantly greater in patients who developed AF compared to those that did not (37 ± 12.2 ml/m2 vs 30 ± 13.4 ml/m2, p=.004), with an area under the receiver operator characteristic curve based on the LAVI of 0.7 (p = 0.004). Kaplan-Meier estimated incidence of AF was significantly greater in patients with LAVI ≥ 30 ml/m2 than LAVI < 30 ml/m2 (66% vs 27%, p=0.004). Risk of incident AF in patients with LAVI > 40 mL/m2 was similar to that of LAVI 30-40 ml/m2 (67% vs 63%, respectively, p=0.97). In multivariable analysis LAVI remained the sole independent predictor of incidence AF after CTI AFL ablation.
LAVI ≥ 30 ml/m2 is associated with significantly increased risk of incident AF following CTI ablation for typical AFL. HATCH <2 was notably not an independent predictor of AF after AFL ablation.
Key Words : Atrial fibrillation, Flutter cavotricuspid, Isthmus ablation, Left atrial volume index.
Dr. Chirag Barbhaiya
Leon H. Charney Division of Cardiology
New York University School of Medicine
550 1st Avenue
New York, NY 10016, USA
Patients undergoing cavotricuspid isthmus (CTI) ablation of typical right atrial flutter (AFL) frequently develop new-onset atrial fibrillation (AF) within three years after ablation 1-3. Previous studies investigating risk factors for incident AF after AFL ablation have not consistently identified LAVI as a predictor and have not commonly included detailed and complete echocardiographic and electrophysiology study data 4-6. Recently, the HATCH score, a risk score incorporating hypertension, age >75 years old, stroke/transient ischemic attack, chronic obstructive pulmonary disease, and heart failure, has been proposed as a predictor of AF after AFL ablation, 7-8 but its utility in clinical decision making remains unclear. Multiple randomized trials have demonstrated the benefit of prophylactic pulmonary vein isolation (PVI) for patients undergoing CTI dependent AFL ablation 9-13. However, prophylactic PVI during AFL ablation is not widely performed, and not included in clinical guidelines 14. Patients at greatest risk of developing incident AF after AFL ablation may derive the greatest benefit from either prophylactic PVI, or intensified monitoring to guide anticoagulation therapy 9-12. We aimed to investigate risk factors, including detailed echocardiography data, and invasive electrophysiology study data, for development of incident AF following AFL ablation.
Retrospective chart review identified 114 consecutive patients without a history of AF or prior cardiac surgery who underwent typical CTI dependent AFL ablation between December 2013 to November 2018, who also had a complete preoperative transthoracic echocardiogram, and at least 1 year of follow-up at our medical center. All available medical records, including baseline characteristics, medication history, ECG, echocardiogram, and electrophysiology study, were reviewed and analyzed by investigators. All available ECGs performed before ablation as well as outpatient telemetry monitoring were reviewed. A total of 21 patients had outpatient telemetry evaluation which consisted of 12 patients with 24 hour monitors, 4 patients with 14 day monitors, 1 patient with a 30 day monitor, and 4 patients with an implanted monitor. The HATCH score was derived by calculation of appropriate variables [hypertension (HTN), Age >75, transient ischemic attack (TIA) /cerebrovascular accident (CVA), chronic obstructive pulmonary disease (COPD) , congestive heart failure (CHF)] 7. Valvular pathology was reported to be present if moderate or greater.
Data collection and analysis were performed according to protocols approved by the NYU Langone Health Institutional Review Board. Surface and intracardiac electrograms (EGMs) were digitally recorded and stored (EP Workmate, Abbott Medical, Inc.). All procedures were performed under conscious sedation after exclusion of left atrial thrombus by transesophageal echocardiography. A 7-French 20-pole catheter (Daig DuoDeca 2-10-2, Abbott Medical, Inc.) was used with the distal poles placed within the coronary sinus and the proximal electrodes located along the tricuspid annulus in the lateral and inferior right atrium. The diagnosis of CTI dependent AFL was confirmed by entrainment or activation mapping at the discretion of the primary operator. Arrhythmia induction by burst pacing was performed in patients presenting in sinus rhythm. 32 patients were found to have atrial tachycardia as their presenting rhythm. Attempted induction of AF was not routinely performed. The primary goal of the procedure was to create a line of bidirectional conduction block in the CTI. Bidirectional block was confirmed by differential pacing with electrodes immediately adjacent to the ablation lesion set. Ablation was performed in each group with a radiofrequency ablation catheter with non-fluoroscopic 3-dimensional mapping (Carto 3, Biosense-Webster, Inc., and NavX, Abbott Medical, Inc.).
Patients were followed for up to 3 years after the date of their procedure. Patient follow-up was censored for the purposes of survival analyses at time of last follow up if less than 3 years after their first procedure. Patients received routine outpatient follow-up at 1 month post-ablation and subsequently at the discretion of their referring cardiologist. Oral anticoagulation was continued for at least 1 month during which a 2 week ambulatory arrhythmia monitor was recommended. The primary outcome was survival free of incident atrial fibrillation after CTI dependent AFL ablation. Diagnosis of AF was defined by the presence of AF >30s duration on ambulatory arrhythmia monitor or implanted device, or on 12 lead ECG.
Categorical data were analyzed across the 2 groups with the chi squared test and were reported as frequencies and percentages. Continuous data were analyzed using the Mann-Whitney U test and were reported as mean + standard deviation. Univariate analyses were performed to evaluate for independent predictors of AF after typical CTI AFL ablation. Univariate and multivariable analyses using the Cox proportional hazard model were performed to evaluate the relationship between LAVI and incidence of AF, and multivariable analysis was adjusted for differences in significant baseline characteristics. A p value criteria of < 0.1 was used to determine which covariates could be included in the multivariable analysis. A receiver operating characteristic (ROC) curve was constructed to test the ability of LAVI to predict new-onset AF and identify an optimal cutoff value. Kaplan-Meier analysis was performed with a log-rank test to determine how LAVI related to the cumulative risk of incident AF. A two-sided P value <0.05 was considered statistically significant. SPSS Statistics software 25.0 (IBM, Armonk, NY) was used for data analysis.
Of 114 patients study patients, 46 patients (40%) were found to develop incident AF during the follow-up period. Of the 32 patients who presented with atrial tachycardia during EPS, all of which were confirmed to be CTI dependent flutter, 12 patients developed AF at follow-up, with an incidence of 37.5%. Of the remaining 82 patients, 34 developed AF, with an incidence of 41%. Baseline characteristics, including age, BMI, and HATCH score were similar amongst patients who developed AF and those that did not develop AF ([Table 1] There was a non-significant trend towards increased incidence of AF in patients with HATCH score ≥ 2 (48% vs 30%, respectively, p= 0.05). There was no significant difference in left ventricular dimension, left ventricular function, or frequency of significant valvular disease between study groups [Table 2]. There was a trend towards increased left atrial diameter in patients that developed incident AF (4.4±0.6 vs 4.1±0.8, p=0.06), while LAVI was significantly greater in patients with incident AF when compared to those that did not (37 ± 12 cm/m2 vs. 30 ±13 cm/m2, p= 0.004). CTI block was achieved in all patients. There were no significant differences in electrophysiology study data between those that developed AF and those that did not [Table 3].
Table 1. Baseline Demographic Data
| Baseline Characteristics |
All patients (N= 114) |
AF on follow up (N= 46) |
No AF on follow up (N= 68) |
p value |
| BSA |
2.07 + 0.2 |
2.1 + 0.3 |
2.1 + 0.2 |
0.9 |
| BMI |
29.9 + 8.0 |
30 + 8.9 |
29 + 7.5 |
0.7 |
| Age (yrs) |
67.5 + 10.5 |
68.9 + 11.8 |
66.5 + 9.5 |
0.1 |
| Male Gender (%) |
103 (90%) |
42(91%) |
61(90%) |
1.0 |
| DM (%) |
21 (18.4%) |
7(15%) |
14(21%) |
0.6 |
| HTN (%) |
68 (59.6%) |
28(61%) |
40 (59%) |
0.9 |
| CAD (%) |
23 (20.1%) |
13(28%) |
10(15%) |
0.1 |
| CVA/TIA (%) |
9(7.9%) |
3(7%) |
6(9%) |
0.7 |
| CHF (%) |
10 (8.8%) |
6(13%) |
4(6%) |
0.2 |
| OSA (%) |
21 (18.4%) |
10 (22%) |
11 (16%) |
0.5 |
| COPD (%) |
18 (15.8%) |
7 (15%) |
11 (16%) |
1.0 |
| NYHA Class (%) |
|
|
|
0.3 |
| I |
31 (27%) |
12 (26%) |
19 (28%) |
|
| II |
30 (26%) |
12 (26%) |
18 (26%) |
|
| III |
7 (6%) |
5 (11%) |
2 (3%) |
|
| CHADS-VASc ≥ 2 (%) |
71 (62.3%) |
28 (61%) |
43(63%) |
1.00 |
| HATCH Score |
1.3 + 1.2 |
1.4 + 1.2 |
1.2 + 1.2 |
0.3 |
| HATCH Score>=2 (%) |
42 (36.8%) |
22 (48%) |
20(30%) |
0.05 |
| Days in Atrial Flutter (days) |
88.1 + 186 |
113.3 + 255 |
70.6 + 116 |
0.9 |
| Medications |
| Beta Blockers (%) |
59 (51.8 %) |
29 (63%) |
30 (44%) |
0.06 |
| CCB (%) |
29 (25.4%) |
10 (22%) |
19 (28%) |
0.5 |
| ACEI/ARB/ARNI (%) |
37 (32.5%) |
13 (28%) |
24(35%) |
0.5 |
| Anticoagulation (%) |
72 (63.2%) |
27 (59%) |
45 (66%) |
0.4 |
ACE/ARB/ARNI= angiotensin converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor; BMI=body mass index; BSA=body surface area; CAD=coronary artery disease; CCB=calcium channel blocker; CHF=congestive heart failure; COPD=chronic obstructive pulmonary disease; CVA/TIA=cerebrovascular accident/transient ischemic event; DM=diabetes mellitus; HTN=hypertension; NYHA=New York Heart Association; OSA=obstructive sleep apnea.
Table 2. Baseline Transthoracic Echocardiography Data
| Echocardiographic Data |
All patients (N= 114) |
AF on follow up (N= 46) |
No AF on follow up (N= 68) |
p value |
| LV EF (%) |
56.7 + 14.0 |
55.7 + 15.4 |
57.4 + 13.1 |
0.99 |
| TTE LA diameter (cm) |
4.2 + 0.7 |
4.4 + 0.6 |
4.1 + 0.8 |
0.06 |
| TTE LA Volume Index (cm/m^2) |
32.8 + 13.2 |
36.8 + 12.2 |
30.2 + 13.4 |
0.004 |
| LVEDD (cm) |
4.6 + 0.9 |
4.8 + 1.1 |
4.5 + 0.7 |
0.07 |
| IV Septum (cm) |
1.2 + 0.2 |
1.2 + 0.2 |
1.1 + 0.2 |
0.6 |
| Inf-Lateral Wall (cm) |
1.2 + 0.5 |
1.2 + 0.7 |
1.1 + 0.2 |
0.4 |
| Aortic root (cm) |
3.4 + 0.4 |
3.5 + 0.3 |
3.3 + 0.4 |
0.09 |
| RAP>5 (mm Hg) |
30 (26.3%) |
14 (36.8%) |
16(29.1%) |
0.7 |
| PASP (mmHg) |
30.1 + 9.1 |
32.2 + 10 |
32.2 + 10 |
0.1 |
| Aortic regurgitation moderate or severe (%) |
6 (5.2%) |
3 (7%) |
3 (4%) |
0.7 |
| Aortic stenosis moderate or severe (%) |
3 (2.6%) |
0 |
3 (4.4%) |
0.3 |
| Tricuspid regurgitation moderate or severe (%) |
10 (8.8%) |
7 (15%) |
3 (4%) |
0.09 |
| Mitral regurgitation moderate or severe (%) |
6 (5.2%) |
3 (7%) |
3 (4) |
0.7 |
| Mitral stenosis (%) |
0 |
0 |
0 |
N/A |
EF= ejection fraction; IV=interventricular; LA=left atrium; LVEDD=left ventricular end diastolic diameter; PASP= pulmonary artery systolic pressure; RAP=right atrial pressure; TTE=transthoracic echo
Study patients were followed for 600 + 405 days after AFL ablation. Intra-procedure cardioversion of atrial fibrillation was required in a total of 5 patients and this did not significantly differ between patients that developed AF and those that did not (9% vs 2%, respectively, p-0.09). There were no post-procedural complications, which included development of hematoma, arteriovenous fistula/pseudoaneurysm, stroke, transient ischemic attack, or cardiac tamponade. One patient developed recurrent CTI dependent atrial flutter at 3 years follow-up. Routine post AFL ablation ambulatory arrhythmia monitoring was completed in 48 patients (42%). Frequency of routine monitoring did not significantly differ between groups that developed AF and those that did not (39% vs.44%, respectively, p= 0.7). Symptom driven ambulatory arrhythmia monitoring was performed in 17 patients (15%), and was performed with similar frequency in patients that developed AF and those that did not (19% vs. 12%, respectively, p= 0.3). Cardiac implantable electronic devices (implantable loop recorder, permanent pacemaker, or implantable cardioverter defibrillator) providing longitudinal arrhythmia monitoring were present in 16 patients (14%), and was present in similar frequency in patients that developed AF and those that did not (15% vs. 13%, respectively, p= 0.8).
Table 3. Procedural Data
| Procedural Data |
All patients (N= 114) |
AF on follow up (N= 46) |
No AF on follow up (N= 68) |
p value |
| Atrial flutter cycle length (ms) |
263 ± 120 |
276 ± 172 |
254 ± 64 |
0.9 |
| RF time (min) |
17.0 ± 9.4 |
17.1 ± 11.6 |
16.7 ± 7.0 |
0.9 |
| Trans-Isthmus Conduction Time (ms) |
158 ± 58 |
161 ± 59 |
156 ± 58 |
0.9 |
| AV node wenckebach (ms) |
441 ± 127 |
428 ± 132 |
451 ± 123 |
0.1 |
| Atrial effective refractory period (ms) |
278 ± 99 |
296 ± 126 |
258 ± 60 |
0.9 |
| AV nodal effective refractory period (ms) |
367 ± 138 |
339 ± 84 |
386 ± 165 |
0.5 |
| Fluoroscopy time (min) |
14.4 ± 11.9 |
15.1 ± 15.8 |
14 ± 8.6 |
0.5 |
| Fluoroscopy dose (mGy) |
302 ± 325 |
274 ± 387 |
321 ± 278 |
0.1 |
| Procedural duration (min) |
80 ± 50 |
83 ± 71 |
78 ± 32 |
0.9 |
AV=atrioventricular; CTI=cavotricuspid isthmus; RF=radiofrequency
[Figure 1] shows the receiver operator curve (ROC) curve for predicting AF after AFL ablation based on LAVI. The area under the curve for LAVI as a predictor of AF was 0.7. A cutoff point of LAVI ≥ 30 ml/m2 derived from ROC curve analysis yielded a sensitivity 71%, specificity 60% for the ability to predict incident AF post-ablation. Univariate analyses identified LAVI >30 and HATCH >2 as statistically significant predictors of incidence of AF after CTI AFL ablation. [Table 4], Multivariable analysis including LAVI ≥ 30 ml/m2 and HATCH ≥ 2 as predictors of AF showed that LAVI ≥ 30 ml/m2 remained the only significant predictor of incidence of AF after CTI AFL ablation (adjusted HR=2.25 [1.14-4.45]. HATCH >2 was notably not an independent predictor of AF after AFL ablation after multivariable analysis. Patients were further stratified according to LAVI < 30 ml/m2 vs. LAVI ≥ 30 ml/m2 and clinical and echocardiographic data were reported for each group [Table 5]-[Table 6]. [Figure 2] demonstrates the Kaplan-Meier estimates of AF-free survival stratified by LAVI ≥ 30 ml/m2, which shows that at a follow up of 3 years, the incidence of AF after CTI AFL ablation is significantly greater in those with LAVI >30 ml/m2 than those with LAVI <30 ml/m2 (66% vs. 27%, p=0.004). A sensitivity analysis of Kaplan-Meier estimates of long term incidence of AF comparing patients with severely increased LAVI ≥ 40 ml/m2 to those with LAVI 30-40 ml/m2 demonstrated no significant difference (67% vs 63%, p= 0.97).
Figure 1. ROC curve for left atrial volume index as a predictor of AF after CTI dependent atrial flutter ablation
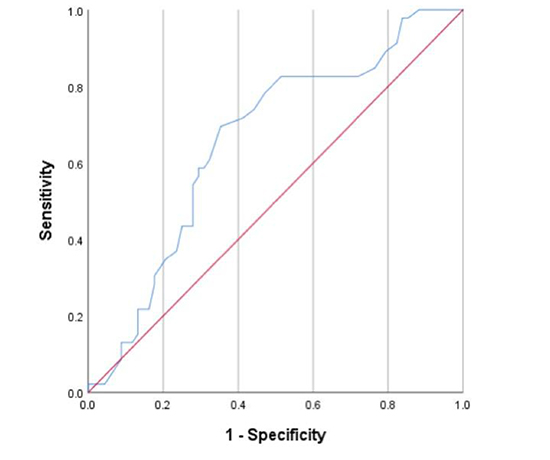
Figure 2. Kaplan-Meier estimates of long-term incidence of AF after cavotricuspid isthmus dependent typical atrial flutter ablation
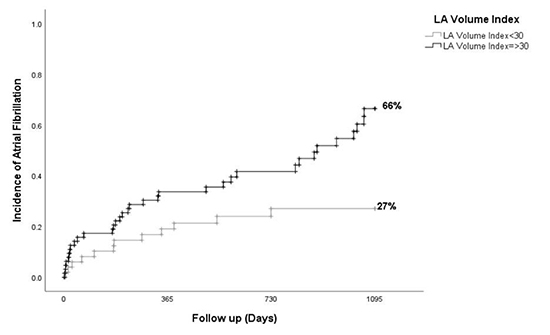
Table 4. Association between left atrial volume index and incidence of atrial fibrillation after CTI dependent atrial flutter ablation
|
Unadjusted Analysis |
Adjusted Analysis |
| Variables |
HR |
95% CI |
p-value |
HR |
95% CI |
p-value |
| LAVI > 30 |
2.54 |
1.31-4.90 |
0.006 |
2.25 |
1.14-4.45 |
0.02 |
| HATCH > 2 |
1.91 |
1.07-3.42 |
0.03 |
1.54 |
0.85-2.82 |
0.16 |
LAVI= Left atrial volume index; HR=Hazard ratio; CI= Confidence interval
Prior studies investigating risk factors for the development of AF after atrial flutter ablation have yielded inconsistent results, [1-2, 4-6,15-16] and effective risk stratification for development of AF after AFL ablation remains an important, unmet clinical need. Our primary findings are as follows: 1) An overall incidence of AF after CTI AFL ablation of 40% in 114 consecutive patients at 3 years follow-up with routine clinical care 2) LAVI was the only independent predictor of AF after CTI AFL ablation and 3) LAVI >30 ml/m2 identified patients significantly more likely to develop AF with a hazard ratio of 2.25, with similar risk of incident AF observed with LAVI 30-40 ml/m2 compared to LAVI ≥ 40 ml/m2.
Table 5. Baseline Demographics data stratified by left atrial volume index
| Baseline Characteristics |
LAVI>30 (N=64) |
LAVI <30 (N= 50) |
p value |
| BSA |
2.0 + 0.3 |
2.1 + 0.2 |
0.1 |
| BMI |
30.3 + 9.4 |
29.2 + 5.3 |
0.6 |
| Age (yrs) |
69 + 10 |
65 + 11.4 |
0.06 |
| Male Gender (%) |
56 (88%) |
47 (94%) |
0.3 |
| DM (%) |
13 (20.3%) |
8 (16%) |
0.6 |
| HTN (%) |
43(67.2%) |
25 (50%) |
0.08 |
| CAD (%) |
13 (20.3%) |
10 (20%) |
1.0 |
| CVA/TIA (%) |
7 (10.9%) |
2 (4%) |
0.3 |
| CHF (%) |
8 (12.5%) |
2 (4%) |
0.2 |
| OSA (%) |
14 (21.9%) |
7 (14%) |
0.4 |
| COPD (%) |
12 (18.8%) |
6 (12%) |
0.4 |
| NYHA Class (%) |
|
|
0.4 |
| I |
14 (21.9%) |
17 (34%) |
|
| II |
14 (21.9%) |
16 (32%) |
|
| III |
5 (7.8%) |
2 (4%) |
|
| CHADS VASc ≥ 2 (%) |
27 (58.7%) |
44 (80%) |
0.015 |
| HATCH Score |
1.5 + 1.3 |
1.1 + 1.2 |
0.003 |
| HATCH Score >2 (%) |
31 (48.4%) |
11 (22%) |
0.006 |
| Days in Atrial Flutter before index procedure (days) |
84 + 207 |
89 + 139 |
0.7 |
| Beta Blockers (%) |
36 (56.3%) |
23 (46%) |
0.3 |
| CCB (%) |
14 (21.9%) |
15 (30%) |
0.4 |
| ACEI/ARB/ARNI (%) |
21 (32.8%) |
16 (32%) |
1.0 |
| Anticoagulation (%) |
43 (67.2%) |
29(58%) |
0.3 |
ACE/ARB/ARNI= angiotensin converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor; BMI=body mass index; BSA=body surface area; CAD=coronary artery disease; CCB=calcium channel blocker; CHF=congestive heart failure; COPD=chronic obstructive pulmonary disease; CVA/TIA=cerebrovascular accident/transient ischemic event; DM=diabetes mellitus; HTN=hypertension; NYHA=New York Heart Association; OSA=obstructive sleep apnea
While incidence of AF after typical AFL ablation in patients with no known history of AF at 3 years follow-up has been reported to be up to 82%, 5 the 40% incidence observed in the present analysis is consistent with that of several prior studies in which incidence of AF after typical AFL ablation was observed to be 25-50% at 3 years follow-up 1,6. Routine, ambulatory arrhythmia monitoring was performed in ~40% of analyzed patients, and monitoring intensity was similar between patients who developed AF and those who did not develop AF.
Table 6. Echocardiographic data stratified by left atrial volume index
| Echocardiographic data |
LAVI > 30 (N=64) |
LAVI <30 (N= 50) |
p value |
| LV EF (%) |
53.1 + 16 |
59.7 + 7.3 |
0.3 |
| TTE LA diameter (cm) |
4.5 + 0.6 |
3.8 + 0.7 |
<0.001 |
| LVEDD (cm) |
4.7 + 1.0 |
4.5 + 0.7 |
0.1 |
| IV Septum (cm) |
1.2 + 0.3 |
1.1 + 0.2 |
0.05 |
| Inf-Lateral Wall (cm) |
1.2 + 0.6 |
1.1 + 0.2 |
0.2 |
| Ao root (cm) |
3.4 + 0.4 |
3.4 + 0.3 |
1.0 |
| RAP>5 (mm Hg) |
19 (30%) |
11 (22%) |
0.5 |
| PASP (mmHg) |
31.2 + 10 |
30 + 7.3 |
0.5 |
| Aortic regurgitation moderate or severe (%) |
4 (6.3%) |
2 (4%) |
0.7 |
| Aortic stenosis moderate or severe (%) |
1 (1.6%) |
2 (4%) |
0.6 |
| Tricuspid regurgitation moderate or severe (%) |
10 (15.6%) |
0 |
0.002 |
| Mitral regurgitation moderate or severe (%) |
6 (9.4%) |
0 |
0.03 |
EF= ejection fraction; IV=interventricular; LA=left atrium; LVEDD=left ventricular end diastolic diameter; PASP= pulmonary artery systolic pressure; RAP=right atrial pressure; TTE=transthoracic echo
Chen et al. investigated predictors of incident AF, including HATCH score, in 216 patients after CTI AFL ablation and found that patients with a HATCH score ≥ 2, and those with increased LA diameter were significantly more likely to develop incident AF 7. The area under the receiver operator curve for HATCH score as a predictor of incident AF after AFL ablation was 0.7. The authors postulated that the HATCH score likely represented those patients with enlarged and remodeled left atriums, however LAVI was not evaluated in that study 7. The significance of the HATCH score was subsequently investigated by Garcia-Seara et al. in 408 patients who underwent typical AFL ablation and it was found that neither a HATCH >2, nor a HATCH > 3 were significant predictors of incidence of AF after typical AFL ablation 8. They did find LA diameter to be significantly associated with incidence of AF, with degree of enlargement correlating with risk of incident AF; however LAVI was not investigated in this study either. Neither HATCH score, nor any other clinical or electrophysiologic parameter have been established as reliable predictors of AF after AFL ablation. Our data are largely consistent with these prior studies suggesting modest utility of HATCH score and LA diameter as predictors of incident AF after typical AFL ablation.
Studies investigating incidence of AF after AFL ablation have not consistently included LAVI as a variable of interest. Limitations of LA size assessment by LA diameter are well recognized 17. Left atrial volume index is calculated via the biplane disk summation technique, which incorporates fewer geometric assumptions than the area-length methods and thus is perceived to be more accurate. Body surface area is also known to largely impact left atrial size; therefore indexing the calculated left atrial volume to body surface area also allows for more accurate interpretation of left atrial volume measurement. In the only prior study that we are aware of that assessed LAVI as a predictor of AF after typical AFL ablation, Lee et al. found a LAVI of 42.6 ml/m2 to be predictive of AF after AFL ablation with a 69% sensitivity and 69.8% specificity 16. The LAVI cutoff proposed by Lee, et al. is considerably greater than our proposed cutoff of 30 ml/m2, and may have been related to greater prevalence of structural heart disease and other comorbidities in their study. Sensitivity analysis in our cohort comparing incidence of AF after CTI AFL ablation in patients with LAVI 30-40 ml/m2 to that of patients with LAVI >40 ml/m2 showed a similarly elevated risk of incident AF in both groups.
Discordance of prognostic significance between LA diameter and LA volume for development of AF was previously shown by Abecasis, et al. in patients who have undergone PVI and CTI ablation for drug resistant AF 18. In this study, LA volume derived from CT scan was a significant predictor of arrhythmia recurrence, however echocardiographic parameters including LA diameter did not have significant predictive value 18. LAVI determined by echocardiography was recently found to be significantly associated with incidence of cardioembolic stroke and incident AF in patients with prior cryptogenic stroke 19. This study result supports the importance of utilizing LAVI in identifying patients at high risk for developing AF post AFL ablation. Typical AFL is strongly associated with coexistent AF, and identification of coexistent AF has significant clinical implications including consideration of anticoagulation. The consistency of association between elevated LAVI and incident AF across study cohorts and disease states provides greater credibility for the potential utility of LAVI for risk-stratification.
Three randomized clinical trials have evaluated prophylactic PVI in patients with typical AFL and no prior history of AF, each of which has yielded results favoring combined PVI and CTI AFL ablation 4-6. These studies of relatively unselected patients undergoing typical AFL ablation found that CTI plus prophylactic PVI ablation resulted in absolute risk reductions for incident AF of 10-28% compared to CTI ablation alone 10. The benefit of prophylactic PVI would be expected to be greatest in patients at greatest risk for development of AF. The established benefit of prophylactic PVI may be substantially greater than previously demonstrated in patients with LAVI of >30 ml/m2, particularly with use of improved ablation techniques for PVI 20.
There were several limitations to this study. Although patients’ medical records including all available ECG documentation were carefully reviewed to exclude the presence of AF before ablation, minimally symptomatic AF may have been present and undiagnosed. Similarly, the frequency of post-ablation AF may be underestimated due to the absence of longitudinal arrhythmia monitoring in all patients. LAVI was also collected directly from echo reports instead of re-calculated, thus the possibility of echocardiographer variability in measuring LAVI is present. Furthermore, the lack of consistent long term ambulatory monitoring post ablation limits this study as well. Additionally, this was a predominantly male patient population, and therefore, these results cannot be generalized to females. Finally, there are limitations to this study that are inherent to its retrospective nature.
LAVI ≥ 30 mL/m2 may provide a simple, intuitive, and clinical meaningful risk stratification for development of AF after CTI AFL ablation. The utility of elevated LAVI in patients undergoing typical AFL ablation to identify patients most likely to benefit from prophylactic PVI or more intensive monitoring prior to discontinuation of anticoagulation requires further evaluation.
LAVI ≥ 30 mL/m2 may provide a simple, intuitive, and clinical meaningful risk stratification for development of AF after CTI AFL ablation. The utility of elevated LAVI in patients undergoing typical AFL ablation to identify patients most likely to benefit from prophylactic PVI ormore intensive monitoring prior to discontinuation of anticoagulation requires further evaluation.