Assessment of left atrial function in patients with paroxysmal, persistent, and permanent atrial fibrillation using two-dimensional strain.
Aleksandra Lenart-Migdalska1, Magdalena Kaźnica-Wiatr 1, Leszek Drabik 12, Klaudia Knap1, Monika Smaś-Suska1, Prof. Piotr Podolec 1, Prof. Maria Olszowska 1
1Department of Cardiac and Vascular Diseases, Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Pradnicka str. 80, 31-202 Krakow, Poland.2Department of Pharmacology, Jagiellonian University Medical College, Grzegorzecka str. 16, 31-531 Krakow, Poland..
Atrial fibrillation (AF) has a progressive nature, leading to structural, functional, and electrical changes in the left atrium (LA). Enhanced response to treatment in patients with AF can be achieved through improved knowledge of atrial structure and a better understanding of its function. The aim of this study was to assess LA strain and its determinants in patients with paroxysmal (PAF), persistent (PsAF), and permanent AF (PmAF).
Fifty-eight patients with registered non-valvular AF were divided into 3 groups depending on the type of AF. The participants underwent transthoracic echocardiography to assess the anatomy and function of heart chambers. Left atrial longitudinal strain (LALS) was measured in four-chamber projections using two-dimensional speckle tracking echocardiography.
Patients with PAF had higher LALS (15.7±12.0) when compared to those with PsAF (4.3±7.9) and PmAF (5.8±7.8, all P=0.003). Multiple linear regression showed that the independent predictors of LALS were diastolic blood pressure (β=0.95, R2=0.88) in the PAF group; left atrial area (β=-0.56) and creatinine (β=-0.63, R2=0.58) in the PsAF group; AF duration (β=0.89) in the PmAF group (R2=0.72).
LA strain has different determinants depending on AF type. LA size, renal function, and AF duration determine LALS in long-lasting AF. LA strain is a simple and accurate technique to estimate LA dysfunction in patients with long-lasting AF.
Key Words : atrial fibrillation, echocardiography, left atrial strain, left atrial function, speckle-tracking.
Correspondence to: Aleksandra Lenart-Migdalska,
Department of Cardiac and Vascular Diseases, Institute of Cardiology, John Paul II Hospital, Pradnicka str.80, 31-202 Krakow, Poland
Atrial fibrillation (AF) is one of the most common cardiac arrhythmias in clinical practice. AF has a progressive nature, leading to structural, functional, and electrical changes in the left atrium. Previous studies have shown that AF begins as paroxysmal in nature, progresses over time, then becomes chronic as the end result [1]. This arrhythmia is characterized by disorganized atrial muscular activation without effective atrial contraction. During AF, the atrial pump function is lost due to asynchronous atrial contraction [2].
Cardiac imaging plays a critical role in the assessment of AF and helps to determine treatment options. Additionally, it helps to identify states that predispose to the development and progression of AF. Cardiac imaging enables early identification of left ventricular (LV) dysfunction or valvular heart disease [3]. It also complements the clinical evaluation, provides AF prognosis, and supports the decision-making process with regard to rhythm strategy (rate control or rhythm control). All of these features make echocardiography the most commonly used imaging technique in the evaluation of AF patients [3-4]. Development of new echocardiographic techniques, such as two-dimensional echocardiographic speckle tracking (STE), have improved the detailed assessment of myocardial properties. Global longitudinal strain, evaluated by STE, is a well-validated parameter used to quantify LV longitudinal function [5] in sinus rhythm and in AF [4,6-7]. Recently, this technique has also been used in the assessment of regional and global left atrial (LA) function [8-10] with good reproducibility [11]. The latest European Association of Cardiovascular Imaging (EACVI)/ European Heart Rhythm Association (EHRA) Expert Consensus Document considers LA strain as a promising method which can be used for indirect measurement of atrial function in AF [6]. The EACVI/ American Society of Echocardiography and Industry Task Force have recently published a document which standardizes LA strain measurements [12].
LA strain measurement has prognostic implications in AF patients [13-14]. Widespread clinical adoption of this approach will require the definition of normal reference ranges in AF patients [15]. Recently, reference ranges for LA strain have been determined in healthy subjects [15-16].
To the best of our knowledge, there have been no reports on LA strain in different types of AF. The aim of this study was to analyze left atrial longitudinal strain (LALS) and estimate its determinants in patients with paroxysmal (PAF), persistent (PsAF), and permanent (PmAF) AF.
Fifty-eight consecutive patients with documented non-valvular AF, admitted to our department between January and July 2017, were enrolled in a prospective study. According to current practice guidelines, we divided patients into 3 groups depending of AF type. Patients with PAF had self-terminating AF episodes lasting up to 7 days. Those with episodes lasting longer than 7 days or requiring cardioversion for termination were in the PsAF group. PmAF was defined as AF which was chronic and accepted by the patient and physician [17].
Exclusion criteria were as follows: left ventricular ejection fraction (LVEF) <30%, severe valvular heart defect, prosthetic heart valves, unstable coronary artery disease (unstable angina pectoris or acute myocardial infarction within the last 30 days), uncontrolled hypertension (≥ 160/100mmHg), stroke (<3 months), recent thromboembolic event (<3 months), congenital heart disease, and chronic kidney disease of stage 4 or more.
We used a standardized questionnaire to collect patient demographic data and information about cardiovascular risk factors and current treatment [18]. The CHA2DS2-VASc score was used for evaluation of risk of stroke or systemic embolism [19]. Bleeding risk was estimated using the HAS-BLED score [17].
Standard echocardiographic evaluation
All patients underwent transthoracic echocardiography using a Philips EPIQ 7 ultrasound machine with synchronous electrocardiogram recording. The measurements were averaged from 3 consecutive cardiac cycles in AF patients while in sinus rhythm and from 5 consecutive cardiac cycles during AF.
LA anatomy was evaluated according to EACVI and EHRA guidelines [6,20-21]. We measured LA anteroposterior diameter (LA AP) using the parasternal long-axis window. LA length, width, area (LAA), and volume (LAV) were determined in the apical 4-chamber (A4C) and apical 2-chamber views. LAV was calculated using the biplane area-length method. Right atrial (RA) longitudinal and short-axis diameters, area, and volume were measured in the A4C view. Measurements were indexed to body surface area (BSA).
LVEF was measured using the biplane Simpson method. LV diastolic function was evaluated by E-velocity deceleration time (EDT), E-wave velocity, e'-velocity, and E/e' ratio. Tricuspid annular plane systolic excursion and peak systolic velocity of the tricuspid annulus were measured to assess right ventricular (RV) function.
Speckle tracking echocardiography
LA strain was measured using two-dimensional STE [12]. Care was taken to obtain true apical images. Five consecutive A4C cardiac cycles were stored with a frame rate of at least 60 frames per second in cine-loop format and then analyzed offline. LA endocardial border was manually traced in A4C, thus delineating a region of interest (ROI). After visually verifying the quality of tracking and eventual manual adjustment of the ROI, the software automatically calculated the average of 7 LA segments and generated time-longitudinal strain curves. Examples of the technique are shown in [Figure 1].
Figure 1. Left atrial longitudinal strain curves.
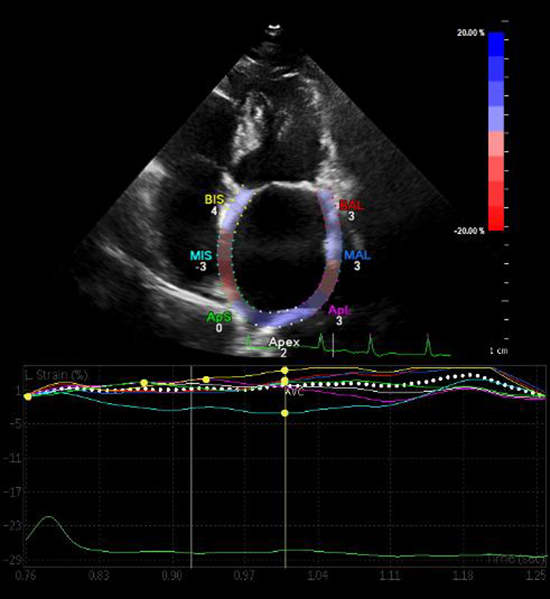
Two-dimensional strain by speckle tracking echocardiography demonstrating left atrial longitudinal strain curves and strain numeric values from the apical four-chamber view. A single cardiac cycle is tracked, the wall of the LA is divided into 7 segments (septal and lateral wall are divided into: basal, mid and apical segments, and apex) which are color coded. The dashed curve represents the average atrial longitudinal strain along the cardiac cycle. Abbreviations: AVC, aortic valve closure.
Intra-observer and inter-observer variability
Inter-observer variability for all measurements was studied in a group of 10 randomly selected subjects. Images were analyzed by 2 independent investigators who were unaware of each other's measurements. Inter-observer variability was determined by repeating the offline measurement of LALS in 10 patients 1 week apart. Variability values were calculated as the absolute difference between corresponding measurements in terms of mean percentage.
The study was powered to have an 80% chance of detecting a 40% difference in LALS between groups at p = 0.05, and was based on a previous study [22]. In order to demonstrate such a difference in LALS or greater, 12 patients were required in each group. Continuous variables were described as mean ± standard deviation or median and interquartile range as appropriate. The Shapiro-Wilk test was used to verify the normality of distribution. Homogeneity of variance was verified using Cochran's test. Means were compared by univariate analysis of variance followed by the Tukey-Kramer test, whereas medians were assessed by the Kruskal–Wallis test and test for multiple comparisons of mean rank. Categorical variables were presented as percentages and compared using the chi-square test and Fisher's exact test. To assess linear dependence between variables, Pearson's correlation coefficient (for normal distribution) or Spearman's rank correlation coefficient (for non-normal distribution) were calculated. To identify independent predictors of LALS, all clinical and laboratory variables which associated with LALS in the univariate model (P > 0.05), but did not significantly correlate (r ≥ 0.5) with another independent variable, were then included in the stepwise multiple linear regression analysis. P values less than 0.05 were considered statistically significant. Data were analyzed using STATISTICA version 13 (Statsoft Inc, Tulsa, OK).
Ethics approval and consent to participate
The study follows the principles of the Declaration of Helsinki. All study procedures involving human participants were performed in accordance with ethical standards of the institutional and national research committee. The study protocol was approved by the local ethics committee. Written informed consent was obtained from patients before enrollment.
The study group was comprised of 58 patients with AF [Table 1], including 16 (27.6%) with PAF, 14 (24.1%) with PsAF, and 28 (48.3%) with PmAF. As shown in [Table 1], the prevalence of cardiovascular risk factors was high, across all AF groups. Most patients (n=49; 84.4%) were at high risk for stroke (CHA2DS2-VASc score ≥2). One patient (1.6%) had low stroke risk (score of 0).
Patients with PsAF had a higher HAS-BLED score when compared with PmAF patients. Patients with PmAF had higher mean heart rate, BSA-indexed LA volume, lower LVEF, EDT, and a trend toward higher E/septale e' when compared with PAF patients [Table 2].
Table 1. General characteristics of the study population
|
Paroxysmal AF
n=16
|
Persistent AF
n=14
|
Permanent AF
n=28
|
p value |
| Demographics |
|
|
|
|
| Age (years) |
69.6 ± 9.4 |
66.9 ± 12.2 |
74.0 ± 8.0 |
0.07 |
| Female sex, n (%) |
13 (76.5) |
5 (38.5) |
14 (50.0) |
0.07 |
| BMI (kg/m2) |
29.3 (27.7-32.4) |
32.3 (28.9-35.3) |
29.4 (26.8 – 35.0) |
0.41 |
| Systolic BP (mmHg) |
122.4 ± 4.7 |
111.7 ± 6.1 |
120.4 ± 3.6 |
0.36 |
| Diastolic BP (mmHg) |
75 (60-80) |
74 (70-80) |
77 (70-80) |
0.45 |
| Heart rate (bpm) |
63 (60-66) |
72 (62-88) |
77 (66-84) |
0.003† |
| Comorbidities and CVD risk factors |
|
|
|
|
| Hypertension, n (%) |
15 (88.2) |
9 (69.2) |
26 (92.3) |
0.15 |
| Hypercholesterolemia, n (%) |
16 (94.1) |
11 (84.2) |
27 (96.4) |
0.42 |
| Diabetes mellitus, n (%) |
6 (35.6) |
1 (7.8) |
11 (39.3) |
0.07 |
| Previous myocardial infarction, n (%) |
2 (11.8) |
2 (15.4) |
0 (0.0) |
0.12 |
| Heart failure, n (%) |
2 (11.8) |
6 (46.2) |
12 (42.9) |
0.06 |
| Previous cerebrovascular events, n (%) |
2 (11.8) |
1 (7.8) |
6 (21.4) |
0.44 |
| Chronic kidney disease, n (%) |
0 (0.0) |
0 (0.0) |
4 (14.3) |
0.10 |
| AF duration (months) |
13 (2-24) |
4 (2-36) |
4 (2-36) |
0.52 |
| CHA2DS2-VASc score |
3.7 ± 1.9 |
2.8 ± 2.0 |
3.9 ± 1.5 |
0.15 |
| HAS-BLED score |
1.9 ± 0.7 |
1.6 ± 1.1 |
2.4 ± 0.8 |
0.03‡ |
| VKA |
3 (17.7) |
0 (0.0) |
8 (28.6) |
0.09 |
| NOAC |
14 (82.3) |
13 (92.3) |
20 (71.4) |
0.09 |
| ACEI |
8 (47.1) |
4 (30.1) |
19 (67.8) |
0.07 |
| Statin |
13 (76.5) |
10 (76.9) |
23 (82.4) |
0.87 |
| Beta-blocker |
17 (88.2) |
12 (92.3) |
22 (78.6) |
0.46 |
| Laboratory parameters |
|
|
|
|
| Creatine (µM) |
77.4 ± 16.2 |
95.9 ± 16.7 |
91.9 ± 19.8 |
0.01* |
| C-reactive protein (mg/L) |
1.2 (0.8-1.8) |
2.3 (0.8-3.6) |
2.1 (1.1-4.1) |
0.22 |
| NT-proBNP (pg/L) |
202 (127-277) |
667 (638-981) |
1259 (794-1960) |
0.07 |
*p <0.05 paroxysmal vs. persistent AF; †p <0.05; paroxysmal vs. permanent AF; ‡ p <0.05 persistent vs. permanent Data are presented as mean ± SD, median (quartile range), and number (percentage) unless otherwise stated. Abbreviations: BMI, body mass index; BP, blood pressure; CVD, cardiovascular; VKA, vitamin K antagonists (warfarin, acenocoumarol); NOAC, non-vitamin K antagonist oral anticoagulants; ACEI, angiotensin-converting enzyme inhibitors; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Table 2. Echocardiographic characteristics of patients
|
Whole group
n=58
|
Paroxysmal AF
n=16
|
Persistent AF
n=14
|
Permanent AF
n=28
|
p value |
| LVEF (%) |
56 (50-60) |
60 (60-65) |
55 (45-62) |
55 (50-60) |
0.01 |
| LAVI (ml/m2) |
47.5 (35.7-53.8) |
35.5 (34.1-36.7) |
47.7 (43.1-53.4) |
51.4 (44.1-55.4) |
0.003 |
| LA enlargement, n (%) |
45 (76.2) |
11 (78.6) |
9 (81.8) |
25 (92.6) |
0.39 |
| MAPSE (mm) |
12 (9.7-16) |
16 (11-10) |
8 (10-13) |
10 (12-14) |
0.09 |
| EDT (ms) |
178 (148-232) |
232 (176-246) |
208 (148-229) |
150 (144-180) |
0.02 |
| E (cm/s) |
90.2 ± 23.2 |
77.4 ± 5.6 |
100.6 ± 6.5 |
94.4 ± 4.7 |
0.02 |
| Septal e'(cm/s) |
8.4 ± 3.0 |
7.7 ±0.8 |
8.5 ± 1.0 |
9.1 ± 0.7 |
0.45 |
| Lateral e'(cm/s) |
11.5 ± 3.8 |
10.4 ± 1.0 |
10.5 ± 1.1 |
12.9 ± 0.9 |
0.10 |
| E/septal e' |
11.9 (8.3-13.3) |
8.1 (7.6-11.8) |
13.2 (10.6-14.1) |
12.0 (9.0-13.3) |
0.07 |
| E/lateral e' |
9.3 (5.7-10.2) |
8.0 (5.7-10.1) |
10.2 (7.0-12.0) |
8.9 (5.6-9.9) |
0.09 |
| E/mean e' |
10.3 ± 3.6 |
9.7 ± 3.5 |
12.2 ± 4.6 |
9.7 ± 2.8 |
0.16 |
| RV S' (cm/s) |
12.1 ± 3.2 |
13.3 ± 1.0 |
12.1 ± 1.0 |
11.4 ±0.8 |
0.34 |
| TAPSE (mm) |
20 (17-24) |
23 (20-30) |
20 (17-25) |
18 (17-22) |
0.24 |
*p <0.05 paroxysmal vs. persistent AF; †p <0.05; paroxysmal vs. permanent AF; ‡ p <0.05 persistent vs. permanent Data are presented as mean ± SD, median (quartile range), and number (percentage) unless otherwise stated. Abbreviations: LAVI, left atrial volume indexed to Body Surface Area; LA enlargement, LAVI >34 ml/m2; LVEF, left ventricular ejection fraction; MAPSE, mitral annular plane systolic excursion; EDT, E-wave deceleration time; E, peak velocity of early filling; Septal e', peak early diastolic septal mitral annular velocity by pulsed tissue Doppler; Lateral e', peak early diastolic lateral mitral annular velocity; Mean e', mean mitral annular peak early diastolic velocity; RV S', peak systolic velocity of the tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion.
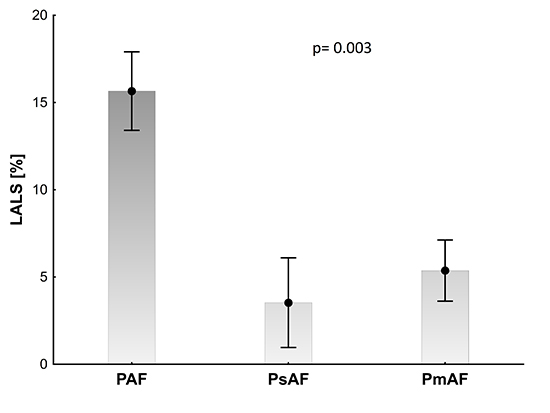
Patients with PAF had higher LALS (15.7±12.0) when compared with PsAF and PmAF patients (4.3±7.9, 5.8±7.8, P=0.003) [Figure 2].
Figure 2. Left atrial longitudinal strain (LALS) in patients with paroxysmal (PAF), persistent (PsAF), and permanent (PmAF) atrial fibrillation are presented as mean values and standard error of mean, p > 0.05 for ANOVA.
In the subgroup with PAF, LALS positively correlated with EDT (r=0.60, p=0.02) and E/lateral e' (r=0.73, p=0.02). In the PsAF group, LALS negatively correlated with creatinine (r=-0.58; p=0.02). In PmAF patients, LALS negatively correlated with CHA2DS2-VASc score (r=-0.47, p=0.01), HAS-BLED score (r=-0.55, p=0.002), E/lateral e' (r=-0.51, p=0.05), and positively correlated with lateral e' (r=0.59, p=0.01), LA width (r=0.40, p=0.04), and AF duration (r=0.89, p=0.02).
In the multiple linear regression model, independent predictors of LALS were as follows: diastolic blood pressure (β=0.95) in the PAF group (R2=0.88); LAA (β=-0.56) and creatinine (β=-0.63) in the PsAF group (R2=0.58); AF duration (β=0.89) in the PmAF group (R2=0.72) [Table 3].
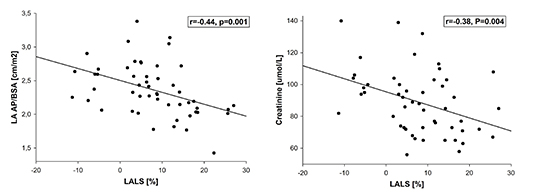
In the entire AF group, LALS was predicted by LA AP/BSA (β=-0.39) and creatinine (β=-0.35 (R2=0.27) [Figure 3].
Table 3. Multiple regression analysis of predictors of LA strain.
| Variables |
AF |
|
Paroxysmal AF
n=16
|
|
Persistent AF
n=14
|
|
Permanent AF
n=27
|
|
|
β (CI 95% ) |
P |
β (CI 95% ) |
P |
β (CI 95% ) |
P |
β (CI 95% ) |
P |
| AF duration (months) |
|
|
|
|
|
|
0.89
(0.24-1.52)
|
0.02 |
| LA AP/BSA (cm/m2) |
-0.39
(-0.14, - 0.63)
|
0.003 |
|
|
|
|
|
|
| LAA (cm2) |
|
|
|
|
-0.56
(-0.16; -0.95)
|
0.01 |
|
|
| Creatinine (µM) |
-0.35
(-0.10, - 0.60)
|
0.008 |
|
|
-0.63
(-0.24; -1.03)
|
0.005 |
|
|
| Diastolic BP (mmHg) |
|
|
0.95
(0.67-1.23)
|
<0.001 |
|
|
|
|
Abbreviations: LA AP, the anteroposterior diameter, parasternal long-axis window; BSA, Body Surface Area; LAA, left atrial area: average of measurements in the apical 4-chamber view and the apical 2-chamber view.
Figure 3. Correlation of left atrial longitudinal strain (LALS) with left atrial anteroposterior diameter - body surface area index (LA AP/BSA) (Panel A) and creatinine (Panel B).
Our study shows that LALS depends on the type of AF, with the lowest values observed in PsAF and PmAF. Depending on AF type, LALS has various levels of association with kidney function, hypertension, and arrhythmia duration.
Decreased LALS values in patients with PsAF and PmAF may reflect progressive LA remodeling and dysfunction, not observed in the beginning of the disease, as is seen in PAF patients. Another finding of our study is an association of LA strain with impaired renal function. LA enlargement (including LAV and LA AP) is frequently observed in patients with chronic kidney disease linked to persistent pressure and volume overload [23]. Therefore, we believe that LALS may reflect chronic exposure to hemodynamic overload in patients with kidney disease. Atrial enlargement is an important marker of LA structural remodeling and a predictor of AF recurrence [24]. Previous prospective studies have shown a strong relationship between LA AP and the risk of new-onset AF [24]. In the Framingham study, a 5-mm increase in LA AP was associated with a 39% higher risk of AF [24-25]. In the Cardiovascular Health Study, subjects in sinus rhythm with LA AP >50 mm had approximately 4 times higher risk of AF [24,26].
LALS may help in the early detection of atrial dysfunction and remodeling and predict AF progression [27] which may lead to new therapies focusing on patients with "early" forms of AF. Atrial remodeling progresses with collagen deposition in the interstitium, with consequent alterations in conduction. Hirose et al. showed that in adults without a history of atrial arrhythmia, a reduction in LA pump function is associated with structural remodeling and initiation of AF development [27-28]. Impaired LA strain indicates reduced LA compliance and impaired reservoir function [4]. LA strain is associated with LA fibrosis, as measured by the degree of delayed-enhancement in cardiac MRI [22,29].
We report that duration of arrhythmia is an independent predictor of LA strain in PmAF patients. The presented data indicates a relationship between AF duration, interstitial atrial remodeling, and LA mechanical dysfunction [27]. Severely impaired LA strain may reflect more advanced LA remodeling [10,30] and predict treatment results. In a study by Tops et al., 63% of participants presented with LA reverse remodeling after catheter ablation (CA) of AF with an accompanying improvement in LA strain [30]. LA strain at baseline was an independent predictor of LA reverse remodeling. Additionally, Parwani et al. demonstrated that LA strain measurement in patients with PsAF may be useful in the selection of patients who are unlikely to benefit from CA [31]. Patients with low LA strain (<10%) had significantly worse results in the long-term follow-up [31]. In AF patients undergoing electrical cardioversion (ECV), LA strain was an independent predictor of restoration and maintenance of sinus rhythm [4,32].
Consistent with other studies, we observed a negative correlation between thromboembolic risk (assessed by CHA2DS2-VASc score) and LALS in PmAF patients. Cameli at al. demonstrated a correlation between reduced LALS, reduced LA emptying velocity, and/or thrombus in patients with PsAF before ECV/CA [33]. Zhu et al. suggested that decreased LA strain in the reservoir phase may become a useful tool for predicting LA appendage stasis in patients with AF [34].
The present study has several limitations. First, size of the investigated groups was limited. However, the number of subjects was sufficient to detect differences between groups based on results of the power calculation. Second, the lack of dedicated, well-established software for acquisition of LA strain meant that we needed to use software designed for LV assessment. Lastly, measurements in the PAF group were made while patients were in sinus rhythm. We cannot completely rule out the impact of sinus rhythm on our results. LALS measurement may facilitate appropriate management strategies in the AF subgroups through improved assessment of LA function, evaluation of LA changes in the course of arrhythmia, and prediction of sinus rhythm return. Our findings should be considered as hypothesis-generating and further confirmation of results in larger prospective investigations is needed.
The study was supported by a research fund from the Jagiellonian University Medical College. The authors report no conflicts of interest.
LALS reflect different levels of LA dysfunction in patients with paroxysmal, persistent, and permanent AF. LA size, renal function, AF duration, and hypertension determine LALS. LA strain assessment in PAF, PsAF, and PmAF may be essential for future research and clinical applications.