Intermittent Nonhabitual Coffee Consumption and Risk of Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis
Jennifer Xu1, Wenjun Fan 1, Matthew J. Budoff 2, Susan R. Heckbert3, Ezra A. Amsterdam4, Alvaro Alonso5, Nathan D. Wong1
1University of California, Irvine Division of Cardiology, C240 Medical Sciences Irvine, CA 92629.2Los Angeles Biomedical Research Institute, 1124 West Carson Street, Torrance, CA 90502.3University of Washington Department of Epidemiology, Box 358085, 1730 Minor Avenue, Suite 1360, Seattle WA 98101.4University of California Davis Medical Center Division of Cardiovascular Medicine, 4860 Y Street #2820, Sacramento, CA 95817.5Department of Epidemiology, Rollins School of Public Health, Emory University, 1518 Clifton Road NE, Atlanta, GA 30322.
Though it is a widely held belief that caffeinated beverages predispose individuals to arrhythmias, it is not clear whether regular coffee consumption is associated with development of atrial fibrillation (AF).
We examined the association between long-term coffee consumption and development of AF in both habitual (≥0.5 cups of daily coffee) and nonhabitual (<0.5 cups/day) drinkers.
A total of 5,972 men and women, aged 45-84 years and without a history of cardiovascular disease at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA) were followed from 2000 to 2014 for incident AF with baseline coffee consumption assessed in 2000-2002 via a Food Frequency Questionnaire and divided into quartiles of 0 cups/day, >0 to <0.5 cups/day, ≥0.5 to 1.5 cups/day, and ≥1.5 cups/day.
Out of the 828 incident cases of AF, intermittent coffee consumption (>0 to 0.5 cups of daily coffee) was associated with a greater risk of incident AF (HR 1.22, 95% CI 1.01-1.48) relative to 0 cups/day in multivariable Cox proportional hazards models after adjustment for numerous AF risk factors. This relation was particularly pronounced in men (adjusted HR=1.36, 95% CI 1.04-1.77). Higher coffee consumption was not associated with AF risk (HR 1.03, 95%CI 0.93-1.14 for ≥0.5 to 1.5 cups/day and 1.05, 95%CI 0.97-1.13 for ≥1.5 cups/day).
While there appears to be no dose-response association between habitual coffee intake and AF risk, we found evidence that intermittent, but not habitual, coffee consumption is associated with a modestly increased risk of incident AF that deserves further study.
Key Words : Coffee, Caffeine, Atrial Fibrillation, Epidemiology, Cardiovascular Disease.
Correspondence to: Nathan D. Wong, PhD Heart Disease Prevention Program, Division of Cardiology University of California, Irvine C240 Medical Sciences Irvine, CA 92629
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia and causes substantial morbidity, mortality and socioeconomic burden [1]. Caffeine consumption is cited as a common trigger for AF episodes but it is not clear whether regular coffee consumption or consumption of large amounts of coffee is actually associated with development of AF [2]. Habitual coffee consumption has been established as having with neutral to beneficial effects on type 2 diabetes, coronary artery disease, congestive heart failure, and stroke [3-7]; furthermore, large observational studies suggest that, compared with non-drinkers, regular coffee drinkers have reduced mortality [8,9]. Interestingly, the effects of coffee have been suggested to be different in habitual drinkers (defined as those who consume more than a half cup of daily coffee) compared to nonhabitual drinkers. In caffeine-naïve subjects, coffee is associated with acute increases in blood pressure but does not affect blood pressure in habitual coffee drinkers [10]. The relationship of coffee to development of AF is not as well characterized. In total three meta-analyses have assessed the association between coffee consumption and development of AF and all have demonstrated no increased risk [11-13]. However, these studies are limited because the division of groups of coffee consumption is inconsistent and may not be able to identify nonhabitual consumers. With consideration of these controversial data, our objective was to clarify the relationship between coffee consumption and the development of AF in both habitual and nonhabitual drinkers from data in the Multi-Ethnic Study of Atherosclerosis (MESA), a large prospective cohort study in the United States.
MESA is a prospective population study whose study methods have been described previously in detail [14]. In brief, between 2000 and 2002, MESA enrolled 6,814 individuals free of cardiovascular disease age 45-84 years from four different race/ethnicities (Caucasian, African-American, Hispanic, and Chinese) from six US field centers. Participants were free of cardiovascular disease at baseline (defined as physician-diagnosed myocardial infarction, angina or nitroglycerin use, stroke or transient ischemic attack, heart failure, current AF or having undergone cardiovascular procedures) and underwent follow-up from 2000 to 2014. Standardized MESA procedures required exclusion of subjects who reported any of these conditions or noninvasive testing indicating a concern for a condition requiring medical follow-up from the participant’s baseline questionnaire.
At the baseline health examination, height, weight, and systolic and diastolic blood pressure were measured. Total cholesterol, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), and triglycerides were measured in nonfasting blood samples. MESA initially defined hypertension using JNC-6 criteria, but in light of recent modifications to the definition by ACC/AHA, hypertension was redefined as taking antihypertensives, systolic blood pressure (BP) ≥130, or diastolic BP ≥80. Participants completed a self-reported questionnaire on highest attained education, cigarette smoking status (never, former, current), total pack year smoking history, alcohol consumption habit (never, former, current), and assessed reported intake of alcoholic drinks (g/day), soda, diet soda, and tea (servings/day).
Baseline coffee consumption was assessed in 2000-2002 via a 120-item Food Frequency Questionnaire (FFQ) that assessed daily intake of foods, beverages, and nutrients in the past year. The questionnaire allowed nine responses that ranged from rare to never, 1-3 per month, once a week, 2-4 per week, 5-6 per week, once a day, 2-3 per day, 4-5 per day, 6+ per day. These responses were converted into daily servings of 0, 0.07, 0.14, 0.43, 0.79, 1, 2.5, 4.5, and 6 cups, respectively. Coffee intake was not differentiated by caffeinated or decaffeinated state and was reported by average serving size as small, medium, or large. A small serving size was characterized as a half cup of coffee, a medium serving was a full cup, and a large serving was 1.5 cups. Using these calculations, we divided average daily coffee consumption into quartiles of 0 cups/day, intermittent nonhabitual (<0.5 cups/day), ≥0.5 to <1.5 cups/day, and ≥1.5 cups/day.
MESA participants or a proxy were contacted by phone every 9 to 12 months to identify all new hospitalizations. Trained staff obtained medical records for all reported hospitalizations and discharge diagnosis International Classification of Diseases, Ninth Revision (ICD-9) codes. AF was identified by: (1) an ICD-9 code for AF (427.31) in any position assigned at hospital discharge, (2) an ICD-9 code for atrial flutter (427.32) in any position assigned at hospital discharge, (3) by study electrocardiogram at a single follow-up visit (2010-2012) (with electrocardiograms reviewed by the study events committee), or (4) for those enrolled in fee-for-service Medicare (55% of the cohort), by an inpatient or outpatient claim with an AF or atrial flutter ICD-9 diagnosis code in any position, using methods adapted from the Cardiovascular Health Study [15]. Hospital discharge diagnosis data and Medicare claims data were available through December 2014. The date of incident AF was defined as the first date AF was noted either by study electrocardiogram or a single ICD-9 code in any position in cohort hospitalization monitoring or Medicare inpatient or outpatient claims data. A review of 16 validation studies determined that the use of the ICD-9 codes to identify AF events has relatively good performance [16].
Participants with preexisting AF (66), incomplete FFQs with >70 questions left blank (282), and lacking clinical covariates (494) were excluded from the analyses leaving 5,972 eligible participants. Baseline characteristics were initially compared across quartiles of coffee consumption using analysis of variance or the Chi-square test of proportions for continuous and categorical variables, respectively.
Multivariable-adjusted Cox proportional hazards regression models were used incorporating time from baseline to incident AF or censoring at death, dropout, or end of the analysis period, December 2014. The lowest approximate quartile corresponding to participants who reported zero coffee consumption was used as the reference category. Nested models with progressive degrees of adjustment were constructed to account for various confounding factors. The first model adjusted for demographic data including age, gender, and race/ethnicity. The second model additionally adjusted for education (less than college degree, college degree, more than college degree), body mass index, systolic BP, diastolic BP, taking antihypertensive medication, diabetes mellitus, LDL-C, and HDL-C. The final model was further adjusted for possible lifestyle confounders including ever/former/current alcohol drinker, daily alcohol intake (g/day), ever/former/current cigarette smoker, pack year smoking history, moderate-vigorous physical activity, and total energy intake, which was calculated from the FFQ. This hierarchial model procedure allowed for examining whether age, gender, and race, first, standard risk factors second, or lifestyle confounders lastly might attenuate any relationships observed between coffee consumption and AF risk. We tested for heterogeneity by including age, gender, education, and race/ethnicity by including interaction of coffee consumption with each covariate, testing statistical significance using the Wald test. A full multivariable model is also provided showing all covariates and their relationships to risk of incident AF.
P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using Stata (version 12.0, StataCorp, College Station, TX, USA).
Characteristics at baseline according to categories of coffee consumption are shown in [Table 1]. Participants were on average 63 ± 10 years old, 47% male, and 40% Caucasian. During 14 years of follow-up a total of 828 incident AF events were identified. The percentage of participants who developed AF in each quartile (0, <0.5, ≥0.5-1.5, and ≥1.5 cups per day of coffee) was 12.0%, 14.6%, 15.0%, and 14.1%, respectively. The frequency of AF episodes is consistent with prior studies that have looked at this population. Those who drank more coffee tended to be male, be of Caucasian race, and have higher education. They were more likely to be cigarette smokers, and consumed more alcohol.
In an unadjusted model looking at the cumulative incidence of AF over time [Figure 1], a Kaplan Meier curve demonstrated an increased risk of AF development in both nonhabitual (<0.5 cups/day) and habitual users (≥0.5 cups/day) compared to participants who drank no coffee.
Figure 1. Kaplan-Meier estimates of the percentages of participants who developed AF.
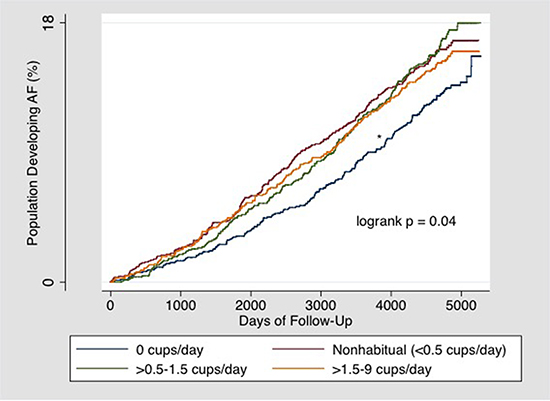
Table 1. Baseline characteristics of participants in the Multi-Ethnic Study of Atherosclerosis (MESA), by approximate quartile of daily coffee consumption
|
0 cups coffee |
Nonhabitual (<0.5 cups coffee) |
≥0.5-1.5 cups coffee |
≥1.5 cups coffee |
|
| Total |
1651 |
1508 |
1378 |
1435 |
|
| Number of AF events |
198 |
221 |
207 |
202 |
|
| % AF events |
12.0% |
14.7% |
15.0% |
14.1% |
|
| Gender |
|
|
|
|
p <0.001 |
| Male |
46.4% |
45.7% |
43.2% |
54.2% |
|
| Female |
53.6% |
54.3% |
56.8% |
45.8% |
|
| Age (years) |
62 (10) |
63 (10) |
64 (10) |
62 (10) |
p=0.001 |
| Race |
|
|
|
|
p <0.001 |
| Caucasian |
29.1% |
25.2% |
42.5% |
66.4% |
|
| Chinese |
19.6% |
16.0% |
5.2% |
2.4% |
|
| African American |
34.5% |
34.5% |
25.2% |
15.1% |
|
| Hispanic |
16.8% |
24.3% |
26.6% |
16.1% |
|
| Education |
|
|
|
|
p <0.001 |
| Less than Bachelor's |
61.7% |
59.1% |
51.2% |
52.4% |
|
| Bachelor's |
12.1% |
16.9% |
24.0% |
18.8% |
|
| Master's or doctorate |
26.2% |
24.0% |
24.8% |
28.8% |
|
| BMI, kg/m2 |
27.8 (5.7) |
28.3 (5.5) |
28.6 (5.3) |
28.6 (5.2) |
p <0.001 |
| Cigarette smoking |
|
|
|
|
p <0.001 |
| Never |
61.5% |
54.9% |
46.3% |
36.0% |
|
| Former |
29.9% |
34.7% |
40.6% |
46.2% |
|
| Current |
8.6% |
10.4% |
13.1% |
17.8% |
|
| Smoking pack-years |
8 (18) |
9 (18) |
11 (18) |
19 (28) |
p <0.001 |
| Diabetes mellitus |
12.5% |
11.0% |
10.2% |
6.6% |
p <0.001 |
| Hypertension (taking antihypertensives,
SBP/DBP ≥130 / ≥80 mmHg)
|
61.5% |
62.9% |
63.6% |
54.9% |
p <0.001 |
| LDL cholesterol, mg/dL |
116 (32) |
118 (32) |
118 (32) |
118 (29) |
p <0.001 |
| Alcohol |
|
|
|
|
p <0.001 |
| Never |
29.1% |
22.1% |
16.0% |
9.3% |
|
| Former |
25.4% |
23.8% |
22.9% |
21.2% |
|
| Current |
45.5% |
54.1% |
61.1% |
69.5% |
|
| Alcohol consumption (g/day) |
2.5 (7.2) |
4.3 (12.3) |
5.4 (12.7) |
9.1 (16.6) |
p <0.001 |
| Soda consumption (cans/day) |
0.39 (1.08) |
0.31 (0.76) |
0.38 (0.85) |
0.42 (0.94) |
p <0.001 |
| Diet soda consumption (cans/day) |
0.39 (1.11) |
0.34 (0.96) |
0.44 (1.05) |
0.60 (1.21) |
p <0.001 |
| Black tea consumption (cups/day) |
0.40 (1.05) |
0.30 (0.75) |
0.25 (0.74) |
0.25 (0.84) |
p <0.001 |
Entries are N (%) for categorical variables and mean (± standard deviation) for continuous variables. Differences between groups were analyzed by analysis of variance (ANOVA) for continuous variables, and by χ2 test for categorical variables.
Table 2. Risk of atrial fibrillation by quartile of coffee consumption in MESA
| Coffee intake (cups/day) |
Total |
0 cups/day |
Nonhabitual (<0.5 cups/day |
≥0.5-1.5 cups/day |
≥1.5 cups/day |
| Total AF events/n (%) |
828/5972 (13.9) |
198/1651 (12.0) |
221/1508 (14.6) |
207/1378 (15.0) |
202/1435 (14.1) |
| Model 1 HR (95% CI) |
|
1 (referent) |
1.23* (1.01-1.49) |
1.06 (0.96-1.17) |
1.07* (1.00-1.15) |
| Model 2 HR (95% CI) |
|
1 (referent) |
1.23* (1.01-1.49) |
1.06 (0.96-1.17) |
1.07* (1.00-1.15) |
| Model 3 HR (95% CI) |
|
1 (referent) |
1.22* (1.01-1.48) |
1.03 (0.93-1.14) |
1.05 (0.97-1.13) |
*p < 0.05 Model 1: adjusted for sex, age, and race/ethnicity Model 2: as model 1 and education, BMI, SBP, DBP, taking antihypertensives, diabetes, LDL, HDL Model 3: as model 2 and never/former/current alcohol drinker, daily alcohol intake, never/former/current tobacco user, pack years, daily soda intake, daily diet soda intake, daily tea intake
After adjustment for demographics, participants in the second (nonhabitual, <0.5 cups/day) quartile had a statistically significant increased risk of AF (HR 1.23, 95% CI 1.01-1.49) The association in the nonhabitual group persisted after adjustment for various cardiovascular and dietary risk factors in three hierarchical models (HR 1.22, 95% CI 1.01-1.48). The association was not dose-dependent as there was no significant difference in AF risk from zero coffee consumption if daily coffee consumption exceeded half a cup per day.
No significant heterogeneity in the association of coffee intake with AF was observed among subgroups defined by age, gender, education, or race/ethnicity (p >0.05 for each interaction). The risk of AF in the nonhabitual group appeared particularly pronounced in males with a HR of 1.36 (95% CI 1.04-1.77) after adjustment for risk factors.
In our full multivariable model [Table 3], in addition to the low dose coffee consumption relation previously noted, age, male sex, body mass index, current smoking, and pack years of smoking were all significantly associated with higher risks for incident AF, while African-American ethnicity and daily tea intake were associated with lower risks for incident AF.
Table 3. Multivariate analysis of demographic, cardiovascular, and dietary risk factors in relation to incident atrial fibrillation
|
Odds ratio |
p-value |
| Age (years) |
1.09 (1.08-1.10) |
<0.001 |
| Gender (M vs. F) |
1.5 (1.26-1.79) |
<0.001 |
| Coffee consumption |
|
|
| <0.5 cups/day vs. none |
1.22 (1.01-1.48) |
0.04 |
| ≥0.5-<1.5 cups/day vs. none |
1.03 (0.93-1.14) |
0.54 |
| ≥1.5 cups/day vs. none |
1.05 (0.97-1.13) |
0.24 |
| Race |
|
|
| Chinese vs. Caucasian |
1.02 (0.78-1.34) |
0.87 |
| African American vs. Caucasian |
0.81 (0.73-0.90) |
<0.001 |
| Hispanic vs. Caucasian |
0.93 (0.86-1.002) |
0.06 |
| Education |
|
|
| Bachelor’s vs. less than Bachelor’s |
1.08 (0.89-1.31) |
0.44 |
| more than Bachelor’s vs. less than |
1.02 (0.93-1.12) |
0.69 |
| Bachelor’s |
1.02 (0.93-1.12) |
0.69 |
| Per Unit BMI (kg/m2) |
1.1 (1.01-1.21) |
0.01 |
| Cigarette smoking |
|
|
| former vs. never |
0.97 (0.79-1.18) |
0.74 |
| current vs. never |
1.23 (1.04-1.45) |
0.02 |
| Smoking pack-years |
1.005 (1.001-1.008) |
0.002 |
| Diabetes mellitus (yes vs. no) |
1.03 (0.96-1.11) |
0.36 |
| LDL cholesterol (per 10 mg/dL) |
0.98 (0.96-1.01) |
0.18 |
| HDL cholesterol (per 10 mg/dL) |
1.03 (0.98-1.08) |
0.29 |
| Alcohol |
|
|
| former vs. never |
1.02 (0.81-1.29) |
0.85 |
| current vs. never |
0.94 (0.85-1.04) |
0.24 |
| Alcohol consumption (g/day) |
1.001 (0.996-1.01) |
0.67 |
| Daily soda intake (cans/day) |
1.001 (0.91-1.10) |
0.97 |
| Daily diet soda intake (cans/day) |
0.99 (0.93-1.07) |
0.87 |
| Daily tea intake (cups/day) |
0.89 (0.81-0.99) |
0.03 |
In this large prospective study, nonhabitual intermittent coffee consumption, which we defined as less than a half cup of daily coffee, was associated with a modest increase in the risk of AF, a finding which persisted in fully-adjusted models. However, there was no increased AF risk if daily coffee consumption exceeded a half cup per day. We have built on the findings of the previous cohort studies, which did not identify a dose-dependent relationship of increasing coffee intake to risk of AF and were encapsulated in a recent review [17]. In 1976, Klatsky et al administered a survey of coffee and tea consumption to 130,000 patients in the Kaiser Permanente health system and followed them until 2008. The study revealed that consumption of four or more cups of coffee per day was associated with an 18% reduction in the risk of hospitalization for arrhythmias, including AF [18]. However, this finding has not been consistent across all studies examining AF incidence and coffee consumption. The Danish Diet, Cancer, and Health cohort study demonstrated that there was no increased risk of AF with consumption of coffee across increasing sextiles of coffee intake in 57,053 participants after a follow-up of 13.5 years until consumption exceeded >6 cups [19]. The Women’s Health Study confirmed these findings in a selected population of healthy 33,738 middle-aged women after a median follow-up of 14.4 years [20]. The Physician’s Health Study was the most recent cohort study that examined this relationship, and found that out of 18,960 male physicians, there was a slight decrease in incident atrial fibrillation in those who drank 1-3 cups of coffee per day after a follow-up of 9 years [21]. It should be noted, also, that the Women’s Health Study analyzed exclusively caffeinated coffee, while the Danish study and the Physician’s Health Study did not distinguish between whether the coffee was caffeinated or decaffeinated. In meta-analyses, cohort studies did not support an association between coffee consumption and development of AF [11-13].
To our knowledge our study is the first to investigate effects of coffee consumption in the nonhabitual group, as prior studies characterize the lowest group of coffee consumption as ranging from less than 1 to 1-4 cups per day [11-13].
Many patients with paroxysmal AF indicate coffee intake and vagal activity as triggering factors for arrhythmia [22,23]. However, the cohort studies mentioned above as well as direct electrophysiologic monitoring indicate that this effect not does appear to be sustained with long-term use. In a study consisting of 1,388 participants undergoing 24-hour Holter monitoring, there was no association noted between higher caffeine intake and atrial or ventricular premature beats [24].
A proposed mechanism for this finding is the development of increased tolerance to the effects of coffee in the acute setting. Caffeine acts on the heart by binding to the A1 and A2 subtypes of the adenosine receptor. In intermittent doses, endogenously released adenosine may shorten atrial refractoriness and predispose to arrhythmias. However, it is possible that habituation could develop with long-term use. Coffee could theoretically confer cardioprotection from AF with habitual intake by attenuating the effects of endogenous adenosine. A controlled trial in dogs found that escalating doses of caffeine decreased propensity for atrial fibrillation [25], and a study of 68 patients in the emergency department who ingested caffeine had decreased responsiveness to a 6 mg bolus of adenosine in the treatment of supraventricular tachycardias [26]. Additionally, coffee has been recently demonstrated to attenuate the affect of coronary vasodilation as detected on myocardial perfusion scintigraphy [27].
There is evidence to suggest that coffee has different effects on cardiac physiology in habitual drinkers compared to nonhabitual drinkers [28]. In a study conducted in Switzerland, nonhabitual coffee drinkers developed acute increases in systolic BP 30-60 minutes after drinking a triple espresso coffee; habitual drinkers did not demonstrate similar findings despite undergoing similar increases in heart rate and sympathetic muscle tone [10]. In the same population, the authors also demonstrated that coffee blunted the blood pressure response to mental stress in habitual, but not in nonhabitual, drinkers [29]. Similar findings have been demonstrated in a study conducted on a Japanese population [30]. There is also evidence to suggest that transient exposure to coffee has an increased risk of triggering myocardial infarction in those with occasional coffee intake compared to nondrinkers; this effect is attenuated with increasing cups [31]. While the definition of “nonhabitual” is not standardized for the time being, most studies characterize it as between the range of less than one cup a day to less than two cups a week.
Our study has strengths and limitations. An important strength is the consistent standardized assessment of coffee intake as well as evaluation of AF. Our study includes both genders, encompasses a broad range of racial and ethnic diversity, and adjusts for additional risk categories related to smoking and alcohol consumption (pack-years and g of alcohol intake) compared to previous cohort studies. Ours is also the first to utilize the modified definition of hypertension of systolic blood pressure (BP) ≥130 mmHg and/or diastolic BP ≥80 mmHg as defined by the ACC/AHA guidelines established in 2017 [32]. With this in mind, prospective studies such as ours cannot infer causality between coffee consumption and risk of AF. FFQs by nature are subject to recall bias and underreporting, and the limitations of nutritional epidemiology have been addressed in many editorials [33,34]. AF events were not classified by duration (paroxysmal versus persistent versus permanent), and the AF incidence is likely underestimated as regular EKGs were not performed in this cohort to determine the presence of asymptomatic AF, which is common in the older population; however, there is no reason to believe any underreporting of AF would be different according to coffee consumption status. Furthermore, because coffee consumption was assessed only at baseline, our study may not reflect long-term behavioral patterns or the effects of changes in coffee consumption. Other limitations include potential residual confounders such as presence of obstructive sleep apnea and sleep patterns, for which data in this cohort was only collected in a limited subset, as well as outcome ascertainment bias in patients with limited access to health care.
We did not directly quantify total caffeine intake in this study. This is because the FFQ did not draw a distinction between decaffeinated and caffeinated coffee, although even had the survey parsed the two, quantifying total caffeine content would be problematical. The caffeine content in a cup of coffee is variable, even if brewed from the same outlet; a standard 8-oz cup of coffee can contain anywhere between 95-200 mg of caffeine [35,36]. Furthermore, it is important to emphasize that caffeine and coffee are not synonymous, and conflation of the two oversimplifies the effect coffee may have on cardiovascular health. In a study of 15 volunteers, intravenous caffeine infusion induced similar increases in muscle sympathetic activity and blood pressure in both habitual and nonhabitual coffee drinkers. However, drinking a cup of coffee increased blood pressure in only the nonhabitual drinkers despite comparable increases of muscle sympathetic activity and plasma caffeine levels in both populations [10].
Drs. Xu, Fan, Heckbert, Amsterdam, Alonso, and Wong have no disclosures to declare. Dr. Budoff is funded by the National Institutes of Health and GE Healthcare, Chicago, IL.
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and grant R01 HL127659 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Dr. Alonso was supported by American Heart Association grant 16EIA26410001. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Our findings support the previously reported studies where there appears to be no dose-response association between coffee intake and AF risk. However, our study finds evidence that intermittent, but not habitual, coffee consumption, which is defined as <0.5 cups of daily coffee, might be associated with a modestly increased risk of incident AF in a healthy population free of cardiovascular disease. Further research is needed to clarify the relationship of coffee consumption, especially intermittent use, with the risk of incident and recurrent AF in regard to the biological mechanism and subgroups in which the effect is particularly pronounced.