Chronic Obstructive Pulmonary Disease and Risk of Atrial Arrhythmias After ST-Segment Elevation Myocardial Infarction
Laurien Goedemans1, Rachid Abou1, José M Montero-Cabezas1, Nina Ajmone Marsan1, Victoria Delgado1, Jeroen J Bax1
1Department of Cardiology, Leiden University Medical Centre, 2300RC Leiden, The Netherlands..
ST-segment elevation myocardial infarction (STEMI) and cardiac arrhythmias frequently occur in patients with chronic obstructive pulmonary disease (COPD). However, little is known about the association of COPD with the occurrence of atrial arrhythmias after STEMI.
This retrospective analysis consisted of 320 patients with first STEMI without a history of atrial arrhythmias, with available 24-hour holter-ECG at 3- and/or 6 months follow-up. In total, 80 COPD patients were compared with 240 non-COPD patients, matched by age and gender (mean age 67±10 years, 74% male). Atrial arrhythmias were defined as: atrial fibrillation/flutter, atrial tachycardia (≥3 consecutive premature atrial contractions (PAC’s)) and excessive supraventricular ectopy activity (ESVEA, ≥30 PAC’s/hour or runs of ≥20 PAC’s).
Baseline characteristics were similar among COPD and non-COPD patients regarding infarct location, β-blocker use and cardiovascular risk profile except for smoking (69% vs. 49%, respectively, p=0.002). Additionally, atrial volumes, LVEF and TAPSE were comparable. During 1 year follow-up, a significantly higher prevalence of atrial tachycardia and ESVEA was observed in patients with COPD as compared to non-COPD patients (70% vs. 46%; p<0.001 and 21% vs. 11%; p=0.024, respectively). In multivariate analysis, COPD was independently associated with the occurrence of atrial arrhythmias (combined) during 1 year of follow-up (HR 3.59, 95% CI 1.78-7.22; p<0.001).
COPD patients after STEMI have a significantly higher prevalence of atrial tachycardia and ESVEA within 1 year follow-up as compared to age- and gender matched patients without COPD. Moreover, COPD is independently associated with an increased prevalence of atrial arrhythmias after STEMI.
Key Words : Chronic obstructive pulmonary disease, Atrial arrhythmias, STEMI.
Correspondence to: Victoria Delgado, MD, PhD; Department of Cardiology, Heart Lung Centre; Albinusdreef 2, 2300 RC Leiden, The Netherlands
Patients with chronic obstructive pulmonary disease (COPD) have an increased risk of cardiac arrhythmias.1, 2 In particular the association between impaired pulmonary function and the development of atrial fibrillation (AF) has been recognised in previous studies.2, 3 In the Atherosclerosis Risk in Communities (ARIC) study, the rate of incident AF increased along with decrease in pulmonary function (measured with forced expiratory volume in 1 second, FEV1).3 Moreover, the independent association between the presence and severity of COPD and the occurrence of AF/atrial flutter and non-sustained ventricular tachycardia (NSVT) has been demonstrated.4
COPD induces hemodynamic changes (pulmonary hypertension, diastolic dysfunction, atrial remodelling), autonomic imbalance, oxidative stress and inflammation that increase the risk of cardiac arrhythmias.2 In addition, the presence of coronary artery disease is frequent in COPD patients and may influence the arrhythmogenic substrate, further increasing the risk of arrhythmias.5 Up to one fifth of the patients with acute myocardial infarction develops AF in the acute setting or during follow-up, doubling the risk of all-cause mortality.6, 7
Despite the known risk of atrial arrhythmias in patients with COPD or acute myocardial infarction, studies addressing a combination of both diseases are scarce. Therefore, the objective of the present study was to evaluate whether COPD is associated with an increased risk of atrial arrhythmias after ST-segment elevation myocardial infarction (STEMI).
From an ongoing clinical registry of ST-segment elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention (PCI) at the Leiden University Medical Center (Leiden, The Netherlands), patients admitted between 2006 and 2012 and with history of chronic obstructive pulmonary disease (COPD) were identified after thorough chart review.8 The diagnosis of COPD was confirmed with pulmonary function tests (if available).8 A control group consisting of STEMI patients without COPD admitted during the same time period was selected. COPD and non-COPD patients were matched by age and gender on a 1:3 basis. All patients were treated according to the institutional STEMI protocol, based on the international STEMI guidelines.9-11 During hospitalization (at least 48 hours), continuous electrocardiographic (ECG) monitoring and transthoracic echocardiography were performed. Guidelines-based medical therapy was initiated. Patients with a previous myocardial infarction, prior documented atrial arrhythmias and/or missing 24-hour Holter-ECG follow-up data were excluded from the present study.
Clinical and echocardiographic data were collected prospectively in the departmental cardiology information system (EPD-vision) and echocardiographic database, respectively. For retrospective analysis of clinically acquired data and anonymously handled, the institutional review board waived the need for patient written informed consent.
Clinical and echocardiographic data
Baseline clinical characteristics included demographic data, cardiovascular risk factors, infarct characteristics consisting of peak levels of creatine kinase (CK) and troponin T, and procedural variables such as culprit vessel and the presence of multi-vessel disease as defined by >50% luminal stenosis in more than one vessel. All echocardiographic data on right and left ventricular and atrial dimensions and function were prospectively measured and analysed (the present study does not concern mere tabulation of data included in clinical echocardiographic reports). Following current guidelines, left atrial volume was indexed to body surface area.12 In addition, color-coded tissue Doppler imaging of the atria was used to assess total atrial conduction time (PA-TDI, time between the onset of the P-wave in the surface ECG and the peak of the A-wave on the TDI velocity recording, a marker of atrial fibrosis, as described before.13 Left ventricular systolic function was evaluated using left ventricular ejection fraction (LVEF) and right ventricular systolic function was evaluated with tricuspid annular plane systolic excursion (TAPSE).12
Follow-up and definitions
Patients were followed-up at the outpatient clinic for a minimum of 1 year. The follow-up included a 24 hour Holter-ECG at 3 and 6 months follow-up. The 12-lead ECG performed at every outpatient clinic visit (within and after 1 year follow-up) was also included in the analysis. Finally, emergency department visits or hospital admissions for atrial arrhythmias were documented.
Three types of atrial arrhythmias were studied: 1) atrial fibrillation (AF) or flutter, 2) atrial tachycardia and 3) excessive supraventricular ectopy activity (ESVEA). Atrial fibrillation or flutter was defined according to current guidelines14, lasting ≥ 30 seconds, either at 12-lead ECG or during 24-hour Holter-ECG monitoring. Atrial tachycardia was defined as a run of ≥3 premature atrial contractions (PACs).15 Premature atrial contractions were characterized by a coupling interval of ≤70% to the preceding QRS complex, based on the mean RR interval of the basic rhythm.15 Finally, ESVEA was defined according to previous literature, as ≥30 PAC per hour or any runs of ≥20 PAC.15 An isolated run of ≥20 PAC’s was considered ESVEA.
The primary endpoint of this study was a composite endpoint of the occurrence of any predefined atrial arrhythmia, during 1 year follow-up after STEMI. Second, the incidence of atrial fibrillation or flutter >1 year after STEMI was registered.
All statistical analyses were performed using SPSS software (version 24, IBM SPSS statistics for windows, Armonk, New York). Categorical data are presented as frequencies and percentages. Comparison of categorical variables between COPD and non-COPD patients was performed using chi-square (χ2) tests or Fisher’s exact test, as appropriate. Continuous data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR), in case of non-normal distribution. Subsequently, continuous data were compared between COPD and non-COPD patients using the unpaired Students-t-test or Mann-Whitney U test, as appropriate.
To identify independent factors associated with the primary endpoint, univariate and multivariate binary logistic regression analysis were performed. Variables with a p-value <0.2 in univariate logistic regression and considered of clinical relevance were included in a multivariate model. Variables with a substantial number of missing values were excluded from the multivariate analysis. The outcomes are presented as odds ratios and associated 95% confidence intervals (CIs). A two-sided p-value of ≤0.05 was considered of statistical significance.
The total study population consisted of 320 STEMI patients (mean age 67±10 years, 74% male), including 80 patients with COPD and 240 age- and sex-matched control patients without COPD. Echocardiography was available for 309 patients (97%). In 276 patients (83%) two 24-hour Holter-ECGs at 3 and 6 months follow-up were available for assessment of atrial arrhythmias. The percentage of patients with two 24-hour Holter-ECGs was similar in COPD and non-COPD patients (86% vs. 83%, respectively, p=0.435). The remaining patients had only one 24-hour Holter-ECG at either 3 or 6 months follow-up.
[Table 1] presents the baseline characteristics of COPD and non-COPD patients. As per study design, no differences in age or gender were present between COPD and non-COPD patients. Furthermore, the distribution of culprit vessels and the presence of multi-vessel disease were similar. Regarding cardiovascular risk factors, patients with COPD were more frequently current or previous smokers (69% vs. 49%, respectively, p=0.002). On echocardiography, patients with COPD had similar atrial volumes, PA-TDI and left and right ventricular systolic function compared to their counterparts [Table 1].
Table 1. Baseline characteristics of patients with and without COPD.
| Variable |
Overall (n=320) |
COPD |
p-value |
| Yes (n=80) |
No (n=240) |
| Age (years) |
67 ± 10 |
70.5 [62 – 77] |
67 [59 – 74] |
0.05 |
| Male (n %) |
236 (74) |
21 (26) |
63 (26) |
1.00 |
| Body surface area (m2) |
1.94 ± 0.2 |
1.96 [1.80 – 2.08] |
1.95 [1.83 – 2.06] |
0.81 |
| Systolic blood pressure (mmHg) |
137 ± 29 |
140 [117 – 165] |
130 [117 – 150] |
0.21 |
| Diastolic blood pressure (mmHg) |
82 ± 18 |
83 [70 – 95] |
80 [70 -90] |
0.15 |
| Heart rate at admission (bpm) |
73 ± 18 |
73.5 [62 – 85] |
70 [60 – 84] |
0.54 |
| Killip class ≥2 (n %) |
12 (4) |
6 (8) |
6 (3) |
0.08 |
| β-blocker use at discharge (n %) |
305 (95) |
74 (93) |
231 (96) |
0.22 |
| eGFR (ml/min/1.73m2) |
85 ± 26 |
88 [69 – 101] |
83 [65 – 102] |
0.41 |
| Peak CK (U/l) |
1298 [603 -2416] |
1253 [551-2125] |
1313 [611 – 2544] |
0.46 |
| Peak Troponin T (µg/l) |
3.16 [1.30-6.47] |
3.1 [1.26 – 5.82] |
3.27 [1.34 – 6.81] |
0.34 |
| Culprit vessel (n %): |
|
|
|
0.29 |
| LAD |
127 (40) |
26 (33) |
101 (43) |
|
| RCA |
143 (45) |
39 (49) |
104 (44) |
|
| LCx |
46 (15) |
14 (18) |
32 (14) |
|
| Multi-vessel disease (n %) |
181 (57) |
42 (53) |
139 (58) |
0.40 |
| Cardiovascular risk factors : |
| Hypertension (n %) |
132 (41) |
32 (40) |
100 (42) |
0.79 |
| Diabetes mellitus (n %) |
33 (10) |
6 (8) |
11 (27) |
0.34 |
| Hypercholesterolemia (n %) |
54 (17) |
14 (18) |
40 (17) |
0.79 |
| Family history of CVD (n %) |
114 (36) |
26 (34) |
88 (37) |
0.59 |
| Current or previous smoking (n %) |
170 (53) |
55 (69) |
115 (49) |
0.002 |
| Echocardiographic parameters: |
| Right atrial area (cm2) |
14.9 ± 3.7 |
14.3 [12 -18] |
14.7 [12.6 – 17] |
0.90 |
| Left atrial volume index (ml/m2) |
23.3 ± 8.3 |
22.5 [16.2 – 26.8] |
22.2 [17.3 – 29.1] |
0.44 |
| PA-TDI duration (ms)* |
109 ± 26 |
109 ± 25 |
108 ± 27 |
0.76 |
| LV end-systolic volume (ml) |
48 [37 – 59] |
47 [38 – 55] |
48 [37 – 62] |
0.66 |
| LV end-diastolic volume (ml) |
93 [74 – 111] |
91 [73 – 111] |
94 [74 – 111] |
0.58 |
| LV ejection fraction (%) |
47 ± 9 |
48 [42 – 53] |
48 [42 – 54] |
0.85 |
| TAPSE (mm) |
17.7 ± 3.7 |
18.3 ± 3.6 |
18.8 ± 3.7 |
0.31 |
CK, creatine phosphokinase; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LAD, left anterior descending coronary artery; LV, left ventricular; RCA, right coronary artery; LCx, left circumflex coronary artery; TAPSE, tricuspid annular plane systolic excursion. *Analysis feasible in 302 patients.
Prevalence of atrial arrhythmias
Table 2 presents the occurrence of atrial arrhythmias at the different time points during follow-up in COPD and non-COPD patients. In particular atrial tachycardia was observed more frequently in patients with COPD at both 24-hour Holter-ECG monitoring time points, when compared to patients without COPD (58% vs 38%; p=0.001 and 57% vs 38%; p=0.005, at 3- and 6 months follow-up, respectively). Additionally, COPD patients had a significantly higher absolute number of PAC’s, both at 3- and 6 months [Table 2]. At 1 year follow-up, atrial tachycardia and ESVEA had occurred more frequently in COPD patients as compared to patients without COPD [Figure 1]. The rate of AF or flutter was low in the overall population (3.8%) and did not differ significantly between groups [Figure 1]. At long-term follow-up (mean time 69.5 ± 39 months), only 5% of the total population had new onset AF, with no significant differences between patients with and without COPD [Table 2]. When analysing the ECG data obtained at emergency room visits, hospital admissions or at the outpatient clinic, the frequency of atrial arrhythmias was similar in COPD and non-COPD patients (9 out of 240 patients without COPD (3.8%) vs. 3 out of 80 patients with COPD (3.8%), p=1.00). Most of these events (67%) were registered prior to the 3-months Holter monitoring (median time interval 3 [IQR 2 – 3,75] months).
Table 2. Occurrence of atrial arrhythmias in patients with and without COPD
|
Overall (n=320) |
COPD |
p-value |
| Yes (n=80) |
No (n=240) |
| Atrial fibrillation < 48 hours (n %) |
18 (6) |
9 (11) |
9 (4) |
0.021 |
| 24-hour Holter-ECG at 3 months (n %) |
316 (99) |
79 (99) |
237 (99) |
1.000 |
| Atrial fibrillation/flutter (n %) |
5 (2) |
3 (4) |
2 (1) |
0.103 |
| Atrial tachycardia (n %) |
135 (42) |
46 (58) |
89 (38) |
0.001 |
| Excessive supraventricular ectopy activity (n %) |
32 (10) |
13 (17) |
19 (8) |
0.032 |
| Premature atrial complexes |
44 [19 – 142] |
65 [29 – 276] |
34 [18 – 119] |
0.017 |
| 24-hour Holter-ECG at 6 months (n %) |
271 (85) |
70 (88) |
201 (84) |
0.645 |
| Atrial fibrillation/flutter (n %) |
5 (2) |
3 (4) |
2 (1) |
0.110 |
| Atrial tachycardia (n %) |
116 (36) |
40 (57) |
76 (38) |
0.005 |
| Excessive supraventricular ectopy activity (n %) |
31 (10) |
11 (16) |
20 (10) |
0.196 |
| Premature atrial complexes |
38 [17 – 141] |
67 [22 – 269] |
33 [15 – 127] |
0.019 |
| Atrial arrhythmia within 1 year (n %) |
177 (55) |
59 (74) |
118 (49) |
<0.001 |
| Atrial fibrillation/flutter > 1 year (n %) |
15 (5) |
3 (4) |
12 (5) |
0.769 |
Continuous variables are presented as median [interquartile range]. COPD; chronic obstructive pulmonary disease, ECG; electrocardiogram.
Figure 1. Prevalence of atrial arrhythmias in patients with (A) and without (B) chronic obstructive pulmonary disease (COPD) after ST-segment elevation myocardial infarction during 1 year follow up. *p<0.001 and §p<0.05, respectively, compared to patients without COPD.
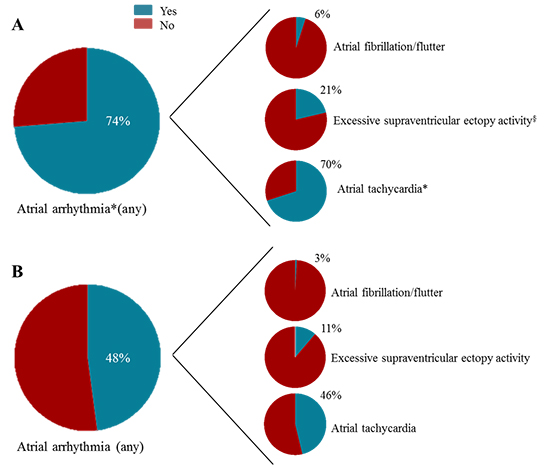
Finally, 177 patients (55%) reached the composite endpoint of any atrial arrhythmia during 1 year follow-up. COPD patients reached more frequently the composite endpoint compared to their counterparts (74% vs 49%, respectively, p<0.001).
Determinants of atrial arrhythmias
[Table 3] presents the clinical and echocardiographic variables with a p-value <0.20 in univariate regression analysis to identify associates of the composite endpoint. Age, male sex, the presence of COPD, kidney function, body surface area and LVEF were significantly associated with the composite endpoint in the univariate analysis. On multivariate analysis, only age (odds ratio 1.13, 95% CI 1.09 – 1.17, p<0.001) and the presence of COPD (odds ratio 3.29, 95% CI 1.64 – 6.58, p=0.001) were independent associates of atrial arrhythmias in this STEMI population [Table 3].
Table 3. Univariate and multivariate determinants of the composite end point.
|
Univariate |
Multivariate |
|
Odds ratio |
95% CI |
p-value |
Odds ratio |
95% CI |
p-value |
| Age, per 1 year increase |
1.14 |
1.11 – 1.17 |
<0.001 |
1.12 |
1.08 – 1.16 |
<0.001 |
| Male sex yes/no |
0.42 |
0.25 – 0.72 |
0.002 |
0.83 |
0.40 – 1.73 |
0.619 |
| COPD yes/no |
2.91 |
1.66 – 5.08 |
<0.001 |
3.29 |
1.64 – 6.58 |
0.001 |
| Hypertension yes/no |
1.52 |
0.97 – 2.39 |
0.07 |
1.32 |
0.75 – 2.35 |
0.339 |
| Killip class ≥2 yes/no |
2.52 |
0.67 – 9.47 |
0.173 |
1.00 |
0.22 – 4.59 |
0.998 |
| Peak troponin level per 1 unit increase |
1.03 |
0.99 – 1.08 |
0.189 |
1.01 |
0.95 – 1.07 |
0.815 |
| eGFR per 1 unit increase |
0.98 |
0.97 – 0.99 |
<0.001 |
0.99 |
0.98 – 1.01 |
0.357 |
| BSA per 1 unit increase |
0.13 |
0.04 – 0.44 |
0.001 |
1.46 |
0.27 – 8.05 |
0.662 |
| β-blocker use yes/no |
0.43 |
0.14 – 1.39 |
0.161 |
0.72 |
0.18 - 2.96 |
0.649 |
| LVEF per 1 unit increase |
0.97 |
0.94 – 0.99 |
0.010 |
0.98 |
0.95 – 1.01 |
0.160 |
| LAVI per 1 unit increase |
1.02 |
0.99 – 1.05 |
0.089 |
1.02 |
0.99 – 1.06 |
0.191 |
BSA, body surface area; CI, confidence interval; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction
The present study demonstrates that patients with COPD have a significantly higher prevalence of atrial tachycardia and ESVEA during 1 year follow-up after STEMI, compared to matched patients without COPD. COPD and increasing age are independently associated with the occurrence of atrial arrhythmias during 1 year follow-up after STEMI.
Cardiac arrhythmias in patients with COPD
Previous studies have shown an independent relationship between COPD and decreased pulmonary function and the occurrence of both supraventricular and ventricular arrhythmias.3, 4 Among AF patients, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) and EURObservational Research Programme Atrial Fibrillation (EORP-AF) registries, including 18,134 and 3,086 patients, respectively, reported an 11% prevalence of COPD in their study populations.16,17 Among patients with COPD, the reported prevalence of cardiac arrhythmias ranges from 5% up to 29%.18 Compared to patients without COPD, the risk of developing cardiac arrhythmias almost doubled in COPD patients (pooled OR 1.94, 95% CI 1.55 – 2.43; p<0.0001).18 In the present study, the incidence of new-onset AF was low in both patient groups (6% vs. 3% for COPD and non-COPD patients, respectively) considering only 1 year follow-up and also at longer term follow-up.
However, almost three-quarters of the COPD patients develop some type of atrial arrhythmia during this short time period compared to only half of the non-COPD patients. The importance of increased atrial ectopic activity for future development of AF was demonstrated recently by Johnson et al.19 A total of 383 patients who underwent 24-hour Holter-ECG recording were evaluated. The number of atrial tachycardia episodes and the presence of ESVEA were independently associated with a 1.99 and 2.66 times increased incidence of AF after a mean follow-up of 10.3 years, respectively.19 The association between PAC count and incident AF was also demonstrated in a larger community-based cohort, analysing 1,260 participants aged 65 years or older.20 The median PAC count of the study population was 2.5 beats/hour (IQR 0.8 – 9.5 beats/hour) and each doubling in PAC count per hour resulted in a 17% increased risk of incident AF during 10 year follow-up.20 These studies accentuate the importance of recording atrial ectopic activity at 24-hour Holter-ECG to identify patients at risk for AF, since PAC’s might be a modifiable risk factor for primary prevention of AF. Ablation of PAC or antiarrhythmic drugs could be initiated at an early stage to reduce the risk of future AF development.21 Furthermore, detection of frequent PAC could lead to more frequent screening and early detection of AF, resulting in timely intervention with anticoagulants reducing the risk of stroke. Even beyond AF, excessive atrial ectopy is associated with an increased risk of ischemic stroke suggesting anticoagulant therapy might be needed even before AF diagnosis is established.22 However, careful considerations should be taken since no evidence is currently available to support these theories.
Pathophysiologic factors responsible for the arrhythmogenic mechanisms in COPD patients are diverse. Both intrinsic factors of the disease itself (i.e. systemic inflammation, oxidative stress) as well as changes in physiology (i.e. hypercapnia, hypoxia, autonomic dysfunction, pulmonary hypertension) could explain this increased arrhythmogenicity. In addition, common concomitant cardiovascular diseases in patients with COPD such as heart failure and coronary artery disease also impact on the occurrence of arrhythmias.2 These factors could potentially lead to atrial structural remodelling or slowing of atrial conduction, increasing the risk of AF. Our results did not show any difference in atrial size or total atrial conduction time, however we did not assess the presence of atrial fibrosis for which cardiac magnetic resonance imaging is the recommended imaging technique.23 This could be of interest for future studies exploring the pathophysiologic mechanisms behind arrhythmias in these patients.
Atrial arrhythmias after acute myocardial infarction
Literature regarding atrial arrhythmias after or during acute myocardial infarction mainly focus on AF or atrial flutter. In the Global Utilization of Streptokinase and TPA (alteplase) for Occluded Coronary Arteries (GUSTO-I) trial, a total of 40,891 STEMI patients were included, with a 10.4% incidence of AF.24 The strongest predictors for incident AF (post-admission) were age, peak CK level, Killip class and heart rate.24 This was later confirmed by the Global Use of Strategies To Open occluded coronary arteries (GUSTO-III) study, regarding only early onset AF <30 days after STEMI.25 In this study, a total population of 13,858 STEMI patients with initial sinus rhythm were included, of whom 906 patients developed AF or atrial flutter during hospitalization (6.5%).25 The independent predictors of new AF in the GUSTO-III study were similar to those described in the GUSTO-I trial, despite the different timing of AF onset in these studies.25 In addition, diabetes, hypertension, prior heart failure and prior myocardial infarction are known to be important predictors of AF in the setting of acute myocardial infarction.6 Although COPD is a recognised risk factor for AF in the general population, data on the influence of COPD as a risk factor for atrial arrhythmias after STEMI are scarce. In the present study, COPD emerges as a strong and independent correlate of the occurrence of atrial arrhythmias within 1 year after STEMI. Variables such as diabetes, hypertension and Killip class at admission were not independently associated with the endpoint in our study. These inconsistencies with the GUSTO-I24 and GUSTO-III25 trials and the review by Schmitt et al.6 could be explained by the use of a combined atrial arrhythmia endpoint (as in the present study) instead of only AF. Also, the present study addressed atrial arrhythmias by 24-hour Holter monitoring several months after STEMI whereas most previous studies evaluated onset of arrhythmias only during hospitalisation.
The insights we provide in our study might create awareness in physicians to consider COPD as a risk factor for atrial arrhythmias after STEMI. Subsequently, this could result in a regular screening program for these patients. The presence of new-onset AF during or shortly after STEMI is associated with higher rates of in-hospital complications (i.e. re-infarction, heart failure, cardiogenic shock), 30-day and 1-year mortality.6, 24, 25 In addition, in 3,220 patients with acute myocardial infarction Jabre et al. demonstrated that patients with late development of AF after acute myocardial infarction (>30 days), have the highest risk of death as compared to patients without new-onset AF (HR 2.58, 95% CI 2.21 – 3.00; p<0.001). Our findings mainly represent arrhythmias at 3- and 6-months follow-up, detecting a substantial incidence of atrial tachycardia and ESVEA in patients with COPD after STEMI. These atrial arrhythmias could lead to incident AF during long-term follow-up, as described before.19, 20 Long-term follow-up for AF/flutter in the present study showed only low prevalence of AF/flutter, although it should be noted that the current study was designed to assess atrial arrhythmias during 1 year follow-up by standardized 24-hour Holter monitoring at regular intervals. Future prospective studies are necessary to provide additional data to support this hypothesis in COPD patients.
Several study limitations should be acknowledged. This is a retrospective study, therefore no causal relationships can be proven. Besides, the single center study design limits the generalizability of the results. Patients were excluded if they were known with atrial arrhythmias prior to the index event (although not all patients had 24-hour Holter monitoring). The presence of arrhythmias was based on 24-hour Holter-ECG monitoring or emergency department visits due to symptoms. Subclinical atrial arrhythmias might therefore be underreported. After 1 year follow-up patients were frequently referred back to their general practitioner or regional hospital for long-term follow-up and therefore, the long-term follow-up data presented may be an underestimation of the true incidence of AF at long-term follow-up. The risk of new-onset COPD with increasing age during follow-up was not considered in the multivariate analysis, although the relatively short follow-up limits the possibility of a significant increase in the incidence of COPD.
Finally, pulmonary function tests were not available for all patients either in their medical history as well as during follow-up, accordingly COPD diagnosis was based on thorough chart review.
COPD patients after STEMI have a significantly higher prevalence of atrial tachycardia and ESVEA within 1 year of follow-up as compared to age- and gender matched patients without COPD. Moreover, COPD was independently associated with the occurrence of atrial arrhythmias 1 year after STEMI. These results indicate that close clinical monitoring might be appropriate in this patient population.