Impact of Denervation by Heart Transplantation on Post-operative Atrial Fibrillation Susceptibility
Neeraj Sathnur1, Jian-Ming Li2, Darshan Krishnappa1, David G Benditt1
1Cardiac Arrhythmia Center, Cardiovascular Division, Department of Medicine.2Division of Cardiology, VA Medical Center, University of Minnesota, Minneapolis, Minnesota.
Atrial fibrillation is common following cardiac and non-cardiac thoracic surgery and is associated with poorer outcomes, including: increased risk of stroke, hemodynamic instability, prolonged hospital stay, and increased mortality. Current understanding suggests that post-op atrial fibrillation results from the interplay of local and systemic operative inflammation, increased sympathetic activity, perhaps the release of free radical species in the perioperative period, and the patient’s underlying cardiac substrate. Cardiac denervation following orthotopic heart transplant (OHT) using modern bicaval techniques presents a unique opportunity to study the relative contribution of the autonomic nervous system to post-op atrial fibrillation susceptibility. Observational studies show a reduced incidence of post-operative atrial fibrillation following orthotopic heart transplant compared to other cardiac and thoracic surgeries. Moreover, comparison of atrial fibrillation rates with double lung transplant recipients suggests that cardiac denervation has a contribution apart from surgical pulmonary vein isolation alone. This report reviews current concepts of the mechanisms of post-op atrial fibrillation with a focus on the role of the autonomic nervous system, the autonomic regulation of the native heart, and evidence regarding the impact of cardiac denervation following OHT.
Key Words : Denervation, Post-Operative Atrial Fibrillation.
Correspondence to: David G Benditt MD, Mail code 508, 420 Delaware St SE, Minneapolis, MN,55455.
Atrial fibrillation is common following both cardiac and non-cardiac thoracic surgery, occurring in 20-60% of patients depending on the population studied and the arrhythmia detection used1-4. Post-operative (post-op) atrial fibrillation is associated with poorer outcomes, including: increased risk of stroke, hemodynamic instability, prolonged hospital stay, and increased mortality5-9. Current understanding suggests that post-op atrial fibrillation results from the interplay of local and systemic operative inflammation, increased sympathetic activity, and perhaps the release of free radical species in the perioperative period; however, the underlying cardiac substrate also governs a patient’s susceptibility.
Cardiac denervation following orthotopic heart transplant (OHT) using current bicaval techniques presents a unique opportunity to study the relative contribution of the autonomic nervous system to post-op atrial fibrillation susceptibility. Comparison with double lung transplant recipients allows the most direct observation of combined surgical pulmonary vein isolation and cardiac denervation, to pulmonary vein isolation (PVI) alone. Although catheter based PVI also disturbs autonomic ganglia its autonomic and electrophysiological effects are substantially less well understood10.
This report reviews current concepts of the mechanisms of post-op atrial fibrillation with a focus on the role of the autonomic nervous system, the autonomic regulation of the native heart, and the impact of cardiac denervation following OHT.
Mechanisms of post-op atrial fibrillation and role of the autonomic nervous system
Post-op atrial fibrillation is a function of i) chronic factors such as age and comorbid conditions that contribute to atrial myopathy and the cardiac substrate’s vulnerability to atrial fibrillation7,11,12 and ii) acute factors related to the physiologic stress of surgery itself. The former has been written about extensively and its review is beyond the scope of this report. Acute factors related to surgery include local and systemic inflammation, increased oxidative stress, and increased sympathetic activation.
The evidence for systemic inflammation as a precipitant of post-op atrial fibrillation is mostly observational, based on several potential contributing factors including: i) the time course of post-op AF correlating with the time course of complement activation and increases in complement-reactive protein13,14, interleukin 215, and interleukin 616; ii) increased white blood cell count being an independent predictor of post-op AF in certain studies17,18; and iii) the observation of increased monocyte activation in patients who develop post-op AF19,20.
Cardiopulmonary bypass is thought to be an important driver of systemic inflammation, through interaction of blood with the circuit machinery triggering the “alternative pathway” of inflammation, and protamine administration triggering the “classical pathway”. Although most studies comparing the rate of post-op AF with “off-pump” and “on-pump” coronary artery bypass surgery failed to show a significant difference in the rate of post-op AF1,21,22, some randomized controlled trials and a meta-analysis of the same have shown a reduction, admittedly small, in post-AF risk in elderly patients (>70 years old) with off-pump, compared to on-pump, coronary artery bypass surgery23. These data suggest that systemic inflammation due to the physiologic stress of surgery makes the larger contribution to provoking post-op AF than does cardiopulmonary bypass.
The data linking local atrial and pericardial inflammation from surgery with post-op AF is conflicting. Two studies examining whether minimally invasive off-pump coronary artery bypass results in less atrial fibrillation than conventional off-pump coronary artery bypass surgery yielded opposing results24,25, indicating perhaps that any trauma to the pericardium results in sufficient pericardial and atrial inflammation such that the amount of direct myocardial manipulation becomes less important.
As free radicals cannot be readily measured in the myocardial tissue of patients during episodes of post-op AF, supportive evidence for oxidative stress as a contributor comes from measurement of lipid peroxidation products and/or observing the effects of antioxidants on the incidence of post-op AF. In this regard, several issues need consideration. First, patients with post-op AF have been reported to have increased systemic and myocardial oxidative stress26. Second, NADPH oxidase activity measured from the right atrial appendage was demonstrated to be the most important independent predictor of post-op AF in patients undergoing coronary artery bypass grafting27. Finally, the administration of antioxidant drugs, specifically ascorbic acid28,29, N-acetylcysteine30, sodium nitroprusside31, and statins32 (which admittedly also have anti-inflammatory properties) have been shown to reduce the incidence of post-op AF.
Increased sympathetic activity is posited to promote post-op AF by increasing myocardial intracellular calcium and consequently atrial ectopy, a trigger of AF, as well as by decreasing action potential duration and reducing the atrial refractory period, which may predispose to localized re-entry and maintenance of atrial fibrillation33,34. Observational evidence supporting the contribution of increased sympathetic activity to post-op AF includes increased norepinephrine levels in patients who develop post-op AF compared to those who do not35 and increased sinus rate and atrial ectopy prior to onset of post-op AF36. Additionally, at least one study reported a decrease in heart rate variability in the hour prior to onset of post-op AF, suggesting that varying autonomic states precede the onset of post-op AF37. On the other hand, a discrepancy between the peak of sympathetic activity (within 24 hours) and somewhat later onset of AF (typically 48-72 hours) post-operatively38 suggests that increased sympathetic activation alone is unlikely to explain all post-op AF and that some interplay with the other proposed mechanisms is responsible.
Clinical trials do demonstrate lower rates of post-op AF in patients receiving beta blockers post-operatively but the discontinuation of pre-op beta blockers in the control arm, with its rebound increase in sympathetic innervation of the heart, may have contributed to increased post-op AF in control groups, thereby overstating the effect of beta blockade in reducing post-op AF39,40. One nonrandomized clinical trial by Melo et al41 examined the impact of ventral cardiac denervation on post-op AF in patients undergoing coronary artery bypass grafting. Denervation was carried out after performing the sternotomy and exposing the heart by excising the fat pads surrounding the vena cava and aorta and main pulmonary artery, thereby removing the nerves entering the hilum along the great vessels. Post-op AF was present in significantly fewer patients who underwent this method of denervation than in the control group (7% vs 27%) but only a third of patients in each group were on telemetry and the success of denervation in the intervention group, as measured by resting heart rate or other measures of sympathetic and parasympathetic innervation, was not reported. Recently there has been interest in invasive and transcutaneous low level stimulation of the vagus nerve to reduce post-op atrial fibrillation although only small trials have been conducted thus far42,43.
Autonomic control of the native heart
The heart is richly innervated and closely regulated by sympathetic and parasympathetic fibers of the autonomic nervous system. Neural control of the heart takes place at extrinsic (extra-cardiac) and intrinsic (within the heart) ganglia. Extrinsic sympathetic innervation originates from the cervical, stellate, and thoracic ganglia while extrinsic parasympathetic input is transmitted from the medullary brainstem by the vagus nerve44,45. However, sympathetic fibers are also present within vagal nerves46. Extrinsic nerves enter the pericardium through the hilum of the heart, its superior posterior region where the parietal pericardium reflects on itself to become the visceral pericardium and where the great vessels pass between the heart and the rest of the thoracic cavity47. Within the pericardium the extrinsic nerves distribute into the intrinsic cardiac autonomic system, which comprises ganglionic plexi innervated with both vagal and adrenergic terminals. These ganglionic plexi Figure 1, located in fat pads around pulmonary vein ostia, the sinus node, and along major coronary artery branches, mediate autonomic inputs that are then transmitted along a network of small nerve fibers48. The ligament of Marshall by the left atrial appendage, too, is richly innervated49. Histologic studies of the distribution of acetylcholinesterase- and tyrosine hydroxylase-positive nerves show a greater density of parasympathetic innervation in the atria and a greater density of sympathetic innervation in the ventricles, although various disease states can cause neural remodeling and alter the distribution of adrenoreceptors50,51.
Figure 1. Posterior schematic view of the heart depicting the general location of the principal atrial and ventricular ganglionic plexi (GPs).
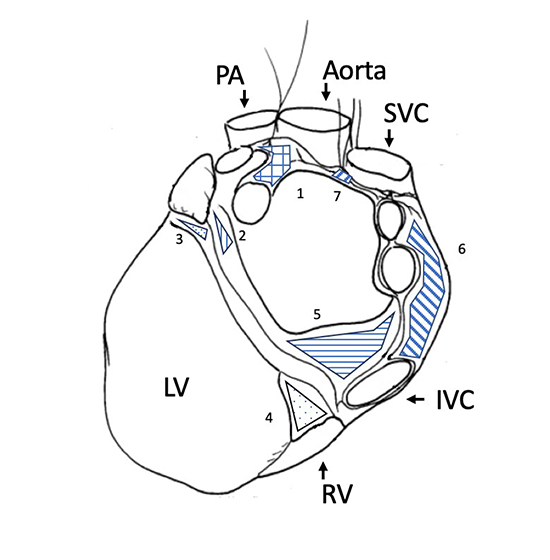
LA=left atrium, RA=right atrium, LV=left ventricle, RV=right ventricle, IVC=inferior vena cava, SVC=superior vena cava, PA=Pulmonary artery, 1. Superior LA GP, 2. Posterolateral LA GP, 3. Obtuse marginal GP, 4. Posterior descending GP, 5. Posteromedial LA GP, 6. Posterior RA GP, 7. Superior RA GP.
Effect of orthotopic heart transplant (OHT) on autonomic inputs to the heart
OHT results in transection of the vagal nerve and the post-ganglionic sympathetic fibers from extrinsic sympathetic ganglia, causing complete denervation of the graft. Axonal degeneration follows, resulting in the disappearance of nerve terminals within transplanted tissue52. Despite loss of the presynaptic neuronal uptake-1 mechanism, which restrains adrenergic activity in the innervated heart through reuptake of norepinephrine into nerve terminals, the graft’s response to circulating catecholamines does not approach the response of the native heart to sympathetic input. Reinnervation occurs variably among patients but 40-70% of recipients demonstrate some degree of reinnervation over time. Sympathetic reinnervation seems not to occur until at least 5-6 months after transplant while parasympathetic reinnervation seems to require at least 1-3 years post-transplant. Even then, reinnervation is incomplete and heterogeneous within the graft53-56.
The loss of autonomic input to the heart has a wide range of immediate effects on cardiac function that variably recover with time as reinnervation restores some autonomic control of the graft. Immediately post-transplant the resting heart rate increases to reflect the intrinsic heart rate i.e., the age-dependent heart rate in absence of autonomic influences57, due to absent vagal and reduced sympathetic input. Three years post-OHT, resting heart rates tend to be lower than in recipients less than three years of transplant, suggesting some return of vagal innervation58. In comparison to control subjects, OHT recipients also show a slower increase in heart rate with exercise and a lower peak heart rate, a result of reduced sympathetic innervation and reliance on circulating catecholamines alone. Some patients demonstrate nearly no rise in heart rate with physical activity59,60. However, several studies show heart rate reserve to increase with time from transplant, suggesting sympathetic reinnervation, and while nearly all recipients in one study had an abnormal heart rate response to exercise two months post-transplant, nearly half had a normalized response by six months61,62. Heart rate variability, another marker of cardiac autonomic regulation, is markedly reduced in early OHT recipients, reliant only on hormonal and internal (i.e., intra-cardiac) control loops63.
Like the heart rate at rest and exercise, heart rate variability (HRV) tends to increase with time. In one study an increase in HRV was observed as early as 15-37 weeks post-transplant in recipients who showed signs of atrial innervation64. However, in most studies evidence of reinnervation did not appear until three years or so post-transplant65,66. The time course and degree of increase in heart rate variability differed among OHT recipients, another sign that reinnervation occurs heterogeneously and to varying extents between patients.
Data concerning the high-frequency power spectrum, a marker of vagal activity, are less consistent with other studies of parasympathetic reinnervation. Many studies show a lower high-frequency power range in OHT recipients with no improvement up to several years post-transplant, suggesting diminished parasympathetic activity despite other parameters indicating evidence of parasympathetic reinnervation66,67. Other studies show an increase in the high-frequency power spectrum with time while others still show no correlation with time following transplant63,65,68.
Other markers of autonomic function including systolic and diastolic blood pressure and catecholamine levels have been studied as well, but have less bearing on the subject of this review. Readers are referred to a paper by Awad et al for a comprehensive review of denervation and reinnervation of the transplanted heart69.
Evidence regarding the impact of cardiac denervation by heart transplant on post-operative atrial fibrillation
OHT recipients have a much lower rate of post-op atrial fibrillation than patients undergoing other cardiac and non-cardiac thoracic surgery. One small initial study in 1995 reported a rate of 18.2% (16 of 88 patients) but multiple larger series since have reported AF rates following transplant of 0.3% (3 of 923 patients), 5.4% (27 of 498), and 7.7% (69 of 892), including those patients who developed AF in close proximity to biopsy-proven rejection, which itself may have been the etiology of AF rather than perioperative factors70-73. This low rate of post-op AF occurs despite pericardiectomy and significant manipulation of the graft, relatively long ischemic times, and post-operative administration of inotropic agents, all of which would be expected to increase the risk of post-op AF. On the other hand, transplanted hearts tend to be young and structurally normal and, perhaps more importantly, steroids and other immunosuppressive medications are routinely administered following transplant.
Additional evidence is available in an observational study by Dizon and colleagues74 examining the rate of post-op atrial fibrillation between 174 consecutive OHT recipients and 122 double lung transplant patients at a single center. Both procedures necessarily result in surgical pulmonary vein isolation but lung transplantation does not transect autonomic inputs to the heart. Only 4.6% of OHT recipients developed post-op AF compared with 18.9% of lung transplant recipients and 19.8% of a comparison group of patients undergoing coronary artery bypass surgery. Both groups received comparable and aggressive immunosuppressive regimens. One difference between the two groups was a higher rate of preoperative beta blocker use in the OHT group (54% vs 13%) although beta blockers are routinely discontinued post-transplant to allow a higher heart rate and contractile function (post-op use was 15% in the OHT group vs 19% in the lung transplant group). No OHT patients received antiarrhythmic agents post-transplant. Both groups had similar rates of inotrope and vasopressor use.
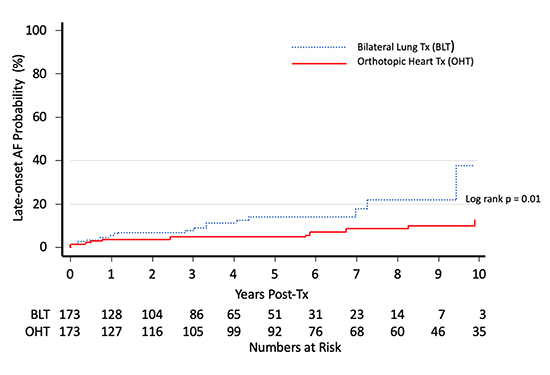
Magruder and colleagues75 also studied a cohort of OHT and double lung transplant recipients and reported a lower incidence of late-onset de novo atrial fibrillation in OHT recipients, up to ten years post-transplant Figure 2.
Figure 2. Probability of late-onset atrial fibrillation among heart vs double lung transplant recipients. Adapted from Magruder et al75.
In another study Noheria and colleagues76 attempted to examine the incremental value of cardiac denervation after OHT by comparing the rate of post-op AF in transplant recipients to a group of patients undergoing non-transplant cardiac surgery with a left atrial Maze lesion set and another group undergoing coronary artery bypass surgery without any pulmonary vein isolation. Although this report found a significantly lower rate of post-op AF in the transplant group (6.5%) than the Maze (22.7%) and CABG (16.4%) groups, the finding could be partly explained by 96% of the Maze group having had a prior history of atrial fibrillation, compared to just 42% in the OHT group (and it is likely that actually far fewer of the transplanted grafts had experienced atrial fibrillation).
Inflammation likely contributes to post-op atrial fibrillation after heart surgery but would be expected to be similar after heart transplantation compared to other cardiac procedures. In this regard, our group examined the impact of pre-op statin use on post-op AF following orthotopic heart transplant and found no difference in the rate of post-op AF between recipients who had or had not been on statin therapy pre-operatively77. This observation can be interpreted to indicate that inflammatory suppression has little to offer in the way of atrial fibrillation suppression in the setting of cardiac denervation with immunosuppressive therapy.
New onset atrial fibrillation after cardiac and non-cardiac thoracic surgery is multifactorial. Nevertheless, the reduction in post-op AF after cardiac denervation by OHT in comparison to surgical PVI from double lung transplant or other cardiac surgeries with or without PVI underscores the role of the autonomic system and sympathetic activation in particular. Further studies are warranted to examine the effectiveness of temporary pharmacologic, device based, or invasive autonomic interventions on reducing post-op AF and its associated poorer post-op outcomes.
- Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M, et al. Atrial fibrillation after coronary artery bypass grafting: does the type of procedure influence the early postoperative incidence? European Journal Of Cardio-Thoracic Surgery. 2001 Apr;19(4):455-9.
- Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004 Apr 14;291(14):1720-9.
- Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996 Aug 1;94(3):390-7.
- Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Risk factors of postoperative atrial fibrillation after cardiac surgery. Journal Of Cardiac Surgery. 2005 Sep-Oct;20(5):425-31.
- Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. Journal Of The American College Of Cardiology. 2004 Mar 3;43(5):742-8.
- Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. European Journal Of Cardio-Thoracic Surgery. 2010 Jun;37(6):1353-9.
- Auer J, Weber T, Berent R, Ng CK, Lamm G, Eber B. Postoperative atrial fibrillation independently predicts prolongation of hospital stay after cardiac surgery. The Journal Of Cardiovascular Surgery. 2005 Dec;46(6):583-8.
- Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. The Annals Of Thoracic Surgery. 2004 Apr;77(4):1241-4.
- Mariscalco G, Engstrom KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. The Annals Of Thoracic Surgery. 2009 Dec;88(6):1871-6.
- Kapa S, Venkatachalam KL, Asirvatham SJ. The autonomic nervous system in cardiac electrophysiology: an elegant interaction and emerging concepts. Cardiology In Review. 2010 Nov-Dec;18(6):275-84.
- Frost L, Molgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PE. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. International Journal Of Cardiology. 1992 Sep;36(3):253-61.
- Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. The Journal Of Thoracic And Cardiovascular Surgery. 1990 Sep;100(3):338-42.
- Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997 Nov 18;96(10):3542-8.
- Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001 Dec 11;104(24):2886-91.
- Hak L, Mysliwska J, Wieckiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). Journal Of Interferon & Cytokine Research. 2009 Jun;29(6):327-32.
- Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003 Sep 9;108 Suppl 1:II195-9.
- Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. Journal Of Cardiothoracic And Vascular Anesthesia. 2006 Feb;20(1):51-6.
- Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. The American Journal Of Cardiology. 2004 May 1;93(9):1176-8.
- Fontes ML, Mathew JP, Rinder HM, Zelterman D, Smith BR, Rinder CS. Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesthesia And Analgesia. 2005 Jul;101(1):17-23.
- Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. The American Journal Of Cardiology. 2010 Jan 15;105(2):186-91.
- Enc Y, Ketenci B, Ozsoy D, Camur G, Kayacioglu I, Terzi S, et al. Atrial fibrillation after surgical revascularization: is there any difference between on-pump and off-pump? European Journal Of Cardio-Thoracic Surgery 2004 Dec;26(6):1129-33.
- Czerny M, Baumer H, Kilo J, Zuckermann A, Grubhofer G, Chevtchik O, et al. Complete revascularization in coronary artery bypass grafting with and without cardiopulmonary bypass. The Annals Of Thoracic Surgery. 2001 Jan;71(1):165-9.
- Panesar SS, Athanasiou T, Nair S, Rao C, Jones C, Nicolaou M, et al. Early outcomes in the elderly: a meta-analysis of 4921 patients undergoing coronary artery bypass grafting--comparison between off-pump and on-pump techniques. Heart (British Cardiac Society). 2006 Dec;92(12):1808-16.
- Scherer M, Sirat AS, Dogan S, Aybek T, Moritz A, Wimmer-Greinecker G. Does totally endoscopic access for off-pump cardiac surgery influence the incidence of postoperative atrial fibrillation in coronary artery bypass grafting? A preliminary report. Cardiovascular Engineering (Dordrecht, Netherlands). 2006 Sep;6(3):118-21.
- Stamou SC, Dangas G, Hill PC, Pfister AJ, Dullum MK, Boyce SW, et al. Atrial fibrillation after beating heart surgery. The American Journal Of Cardiology. 2000 Jul 1;86(1):64-7.
- Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, et al. Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study. The Annals Of Thoracic Surgery. 2007 Oct;84(4):1166-72; discussion 72-3.
- Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. Journal Of The American College Of Cardiology. 2008 Jan 1;51(1):68-74.
- Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circulation Research. 2001 Sep 14;89(6):E32-8.
- Eslami M, Badkoubeh RS, Mousavi M, Radmehr H, Salehi M, Tavakoli N, et al. Oral ascorbic acid in combination with beta-blockers is more effective than beta-blockers alone in the prevention of atrial fibrillation after coronary artery bypass grafting. Texas Heart Institute Journal. 2007;34(3):268-74.
- Erdogan O. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. European Heart Journal. 2008 Jun;29(12):1591; author reply
- Cavolli R, Kaya K, Aslan A, Emiroglu O, Erturk S, Korkmaz O, et al. Does sodium nitroprusside decrease the incidence of atrial fibrillation after myocardial revascularization?: a pilot study. Circulation. 2008 Jul 29;118(5):476-81.
- Liakopoulos OJ, Choi YH, Kuhn EW, Wittwer T, Borys M, Madershahian N, et al. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. The Journal Of Thoracic And Cardiovascular Surgery. 2009 Sep;138(3):678-86 e1.
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. The American Journal Of Physiology. 1997 Aug;273(2 Pt 2):H805-16.
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 Jun;2(6):624-31.
- Kalman JM, Munawar M, Howes LG, Louis WJ, Buxton BF, Gutteridge G, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. The Annals Of Thoracic Surgery. 1995 Dec;60(6):1709-15.
- Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. The American Journal Of cardiology. 1998 Jul 1;82(1):22-5.
- Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. Journal Of The American College Of Cardiology. 2003 Oct 1;42(7):1262-8.
- Hogue CW, Jr., Hyder ML. Atrial fibrillation after cardiac operation: risks, mechanisms, and treatment. The Annals Of Thoracic Surgery. 2000 Jan;69(1):300-6.
- Ali IM, Sanalla AA, Clark V. Beta-blocker effects on postoperative atrial fibrillation. European Journal Of Cardio-Thoracic Surgery. 1997 Jun;11(6):1154-7.
- White HD, Antman EM, Glynn MA, Collins JJ, Cohn LH, Shemin RJ, et al. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary artery bypass surgery. Circulation. 1984 Sep;70(3):479-84.
- Melo J, Voigt P, Sonmez B, Ferreira M, Abecasis M, Rebocho M, et al. Ventral cardiac denervation reduces the incidence of atrial fibrillation after coronary artery bypass grafting. The Journal Of Thoracic And Cardiovascular Surgery. 2004 Feb;127(2):511-6.
- Akdemir B, Benditt DG. Vagus nerve stimulation: An evolving adjunctive treatment for cardiac disease. Anatolian Journal Of Cardiology. 2016 Oct;16(10):804-10.
- Andreas M, Arzl P, Mitterbauer A, Ballarini NM, Kainz FM, Kocher A, et al. Electrical Stimulation of the Greater Auricular Nerve to Reduce Postoperative Atrial Fibrillation. Circulation Arrhythmia And Electrophysiology. 2019 Oct;12(10):e007711.
- Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. The Journal Of Physiology. 1957 Jan 23;135(1):182-205.
- Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anatomy And Embryology. 2005 Jul;209(6):425-38.
- Seki A, Green HR, Lee TD, Hong L, Tan J, Vinters HV, et al. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm. 2014 Aug;11(8):1411-7.
- Pauza DH, Pauziene N, Tamasauskas KA, Stropus R. Hilum of the heart. The Anatomical Record. 1997 Jul;248(3):322-4.
- Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. The Anatomical record. 1997 Feb;247(2):289-98.
- Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. Journal Of The American College Of Cardiology. 2000 Oct;36(4):1324-7.
- Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Research In Cardiology. 2001 Nov;96(6):528-38.
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart And Vessels. 2003 Mar;18(1):32-9.
- Norvell JE, Lower RR. Degeneration and regeneration of the nerves of the heart after transplantation. Transplantation. 1973 Mar;15(3):337-44.
- Bernardi L, Valenti C, Wdowczyck-Szulc J, Frey AW, Rinaldi M, Spadacini G, et al. Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation. 1998 Apr 14;97(14):1368-74.
- Buendia-Fuentes F, Almenar L, Ruiz C, Vercher JL, Sanchez-Lazaro I, Martinez-Dolz L, et al. Sympathetic reinnervation 1 year after heart transplantation, assessed using iodine-123 metaiodobenzylguanidine imaging. Transplantation Proceedings. 2011 Jul-Aug;43(6):2247-8.
- Lovric SS, Avbelj V, Trobec R, Zorman D, Rakovec P, Hojker S, et al. Sympathetic reinnervation after heart transplantation, assessed by iodine-123 metaiodobenzylguanidine imaging, and heart rate variability. European Journal Of Cardio-Thoracic Surgery. 2004 Oct;26(4):736-41.
- Parry DS, Foulsham L, Jenkins G, Wharton J, Marron K, Banner N, et al. Incidence and functional significance of sympathetic reinnervation after cardiac transplantation. Transplantation Proceedings. 1997 Feb-Mar;29(1-2):569-70.
- Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovascular Research. 1970 Apr;4(2):160-7.
- Goncalvesova E, Micutkova L, Mravec B, Ksinantova L, Krizanova O, Fabian J, et al. Changes in gene expression of phenylethanolamine N-methyltransferase in the transplanted human heart. Annals Of The New York Academy Of Sciences. 2004 Jun;1018:430-6.
- Braith RW, Plunkett MB, Mills RM, Jr. Cardiac output responses during exercise in volume-expanded heart transplant recipients. The American Journal Of Cardiology. 1998 May 1;81(9):1152-6.
- Pflugfelder PW, Purves PD, McKenzie FN, Kostuk WJ. Cardiac dynamics during supine exercise in cyclosporine-treated orthotopic heart transplant recipients: assessment by radionuclide angiography. Journal Of The American College Of Cardiology. 1987 Aug;10(2):336-41.
- Buendia Fuentes F, Martinez-Dolz L, Almenar Bonet L, Sanchez-Lazaro I, Navarro Manchon J, Sanchez-Gomez JM, et al. Normalization of the heart rate response to exercise 6 months after cardiac transplantation. Transplantation Proceedings. 2010 Oct;42(8):3186-8.
- Ferretti G, Marconi C, Achilli G, Caspani E, Fiocchi R, Mamprin F, et al. The heart rate response to exercise and circulating catecholamines in heart transplant recipients. Pflugers Archiv : European Journal Of Physiology. 2002 Jan;443(3):370-6.
- Toledo E, Pinhas I, Aravot D, Almog Y, Akselrod S. Functional restitution of cardiac control in heart transplant patients. American Journal Of Physiology Regulatory, Integrative And Comparative Physiology. 2002 Mar;282(3):R900-8.
- Smith ML, Ellenbogen KA, Eckberg DL, Sheehan HM, Thames MD. Subnormal parasympathetic activity after cardiac transplantation. The American Journal Of Cardiology. 1990 Nov 15;66(17):1243-6.
- Beckers F, Ramaekers D, Speijer G, Ector H, Vanhaecke J, Verheyden B, et al. Different evolutions in heart rate variability after heart transplantation: 10-year follow-up. Transplantation. 2004 Nov 27;78(10):1523-31.
- Halpert I, Goldberg AD, Levine AB, Levine TB, Kornberg R, Kelly C, et al. Reinnervation of the transplanted human heart as evidenced from heart rate variability studies. The American Journal Of Cardiology. 1996 Jan 15;77(2):180-3.
- Keeley EC, Toth ZK, Goldberg AD. Long-term assessment of heart rate variability in cardiac transplant recipients. The Journal Of Heart And Lung Transplantation. 2000 Mar;19(3):310-2.
- Uberfuhr P, Frey AW, Fuchs A, Paniara C, Roskamm H, Schwaiger M, et al. Signs of vagal reinnervation 4 years after heart transplantation in spectra of heart rate variability. European Journal Of Cardio-Thoracic Surgery. 1997 Dec;12(6):907-12.
- Awad M, Czer LS, Hou M, Golshani SS, Goltche M, De Robertis M, et al. Early Denervation and Later Reinnervation of the Heart Following Cardiac Transplantation: A Review. Journal Of The American Heart Association. 2016 Nov 1;5(11).
- Cohn WE, Gregoric ID, Radovancevic B, Wolf RK, Frazier OH. Atrial fibrillation after cardiac transplantation: experience in 498 consecutive cases. The Annals Of Thoracic Surgery. 2008 Jan;85(1):56-8.
- Cui G, Tung T, Kobashigawa J, Laks H, Sen L. Increased incidence of atrial flutter associated with the rejection of heart transplantation. The American Journal Of Cardiology. 2001 Aug 1;88(3):280-4.
- Khan M, Kalahasti V, Rajagopal V, Khaykin Y, Wazni O, Almahameed S, et al. Incidence of atrial fibrillation in heart transplant patients: long-term follow-up. Journal Of Cardiovascular Electrophysiology. 2006 Aug;17(8):827-31.
- Pavri BB, O'Nunain SS, Newell JB, Ruskin JN, William G. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. Journal Of The American College Of Cardiology. 1995 Jun;25(7):1673-80.
- Dizon JM, Chen K, Bacchetta M, Argenziano M, Mancini D, Biviano A, et al. A comparison of atrial arrhythmias after heart or double-lung transplantation at a single center: insights into the mechanism of post-operative atrial fibrillation. Journal Of The American College Of Cardiology. 2009 Nov 24;54(22):2043-8.
- Magruder JT, Plum W, Crawford TC, Grimm JC, Borja MC, Berger RD, et al. Incidence of late atrial fibrillation in bilateral lung versus heart transplants. Asian Cardiovascular & Thoracic Annals. 2016 Oct;24(8):772-8.
- Noheria A, Patel SM, Mirzoyev S, Madhavan M, Friedman PA, Packer DL, et al. Decreased postoperative atrial fibrillation following cardiac transplantation: the significance of autonomic denervation. Pacing and clinical electrophysiology. 2013 Jun;36(6):741-7.
- Krishnan B, Vakil KP, Sankar A, Duprez D, Benditt DG. Impact of pre-operative statin use on risk of mortality and early atrial fibrillation after heart transplantation. Clinical Transplantation. 2016 May;30(5):628-32.