Atrial Septal Defect and Atrial Fibrillation:
The Known and Unknown
George E.Blake, MD, Dhanunjaya Lakkireddy,MD
Mid America Cardiology @ University of Kansas Hospital, Kansas City, KS.
Atrial fibrillation (AF) is a common complication in patients with atrial septal defects (ASDs). The link between AF and ASD is fairly complex and entails modifications in electrophysiologic, contractile and structural properties, at the cellular and tissue level, of both atria, mainly due to chronic atrial stretch and dilation. Surgical repair or percutaneous closure of ASDs are equally effective in reducing mortality and symptoms but limited in preventing or curbing AF, unless combined with an arrhythmia-specific procedure. Transesophageal echocardiography (TEE) and intracardiac echocardiography (ICE) have improved the safety and success of the above procedures. Finally, clearer understanding of the pathophysiology of AF in patients with ASD (and CHF, in general) has led to target-specific advances in medical management.
Correspondence to: Dr. Dhanunjaya Lakkireddy MD, FACC, Director – Center for Excellence in Atrial Fibrillation, Bloch Heart Rhythm Center, University of Kansas Hospitals, Kansas City.
AF is the most common sustained arrhythmia in clinical practice currently affecting approximately 2.2 million people in the United States with an estimate of more than 5.6 million, 50% of which aged 80 years or older, by the year 2050. The prevalence of AF directly correlates with age, with only 0.1% of adults younger than 55 years but 9.0% of adults 80 years or older affected.1 Age-standardized prevalence of AF is higher among men than women and among patients with essential hypertension, ischemic or valvular heart disease, left atrial (LA) enlargement, congestive heart failure and diabetes, with cardiac failure and rheumatic heart disease being the most powerful predictive precursors.2-13 AF is a very costly disease with significant impact on public health. The number of hospitalizations for AF has dramatically increased in the recent years with a subsequent rise in health care costs.14-16 The development of AF places patients at significant risk for cardioembolic strokes, steadily increasing with age from 6.7% for ages 50 to 59 years to 36.2% for ages 80-89 years5 and doubles their overall mortality.8 In the subgroup of patients with ASD the incidence of atrial flutter (AFL) and AF has been reported to be as high as 15-40% in the 30-35 year olds and carries ominous prognosis7,17
ASDs are quite common representing 10% of all cardiac malformations at birth, 12% in children and 15% at clinics for children and adults.18 There are 3 major types of ASDs: ostium secundum, ostium primum and sinus venosus defects. The ostium secundum is a true defect of the atrial septum and is located in the region of the fossa ovalis. The ostium primum defect belongs to the atrioventricular (AV) septal defects (also known as endocardial cushion defects), which may include a large ventricular septal defect and a common AV valve in its complete form. The sinus venosus defect is usually located at the junction of the right atrium (RA) and superior vena cava and almost always associated with partial anomalous pulmonary venous return. Two less common types of ASDs include the inferior vena cava form of sinus venosus defect and the coronary sinus septal defect.19 With any ASD, the direction and amount of flow across the defect depends on the size of the defect and the diastolic properties of the two ventricles, which at birth are approximately equal and hence there is minimal net flow across the defect. As pulmonary arterial pressure falls, the right ventricle (RV) atrophies and becomes more distensible, while the left ventricle (LV) hypertrophies and becomes less compliant, due to increased systemic vascular resistance. This is the time when shunting of blood from the LA to the RA begins overloading the RV and shifting the interventricular septum to the left, decreasing the LV preload. The renis-angiotensin-aldosterone (RAA) system is thus activated increasing the intravascular volume and further increasing the pulmonary blood flow, eventually causing RA, LA and RV dilatation, pulmonary hypertension, right heart failure and flow reversal across the interatrial defect with significant hypoxia and cyanosis (Eisenmenger syndrome).20 The most common initial presenting symptoms of ASD are exertional dyspnea or fatigue. Patients may then develop AF, decompensated right heart failure, occasionally, paradoxical embolus or transient ischemic attack, and finally, cyanosis (especially with inferior sinus venosus defect or right-to-left shunts).19,21-25 Roesler in 1934 was the first to describe the natural history and clinical features of the disease based on 62 post mortem cases. Mean age of death was 36 years and usually resulted from heart failure, often associated with AF.26 Campbell noticed that the mortality rates were quite low for the first two decades of life (0.6 to 0.7% per year) but rose significantly with subsequent decades (2.7% to 4.5% to 5.4% to 7.5% per year).18
AF Induction and Maintenance in ASD Patients
The link between AF and ASD is relatively complex. Changes in atrial size and pressures have been shown to induce and maintain atrial tachyarrhythmias by various mechanisms. The effects of acute atrial stretch on atrial electrophysiology are widely divergent. Sideris et al found that an increase in right atrial pressure by acute volume overload significantly prolonged right atrial effective refractory period (AERP), interatrial conduction time and increased the propensity of the atria to fibrillate by rapid atrial pacing (RAP).27 Other investigators, however, concluded that acute atrial stretch highly correlated with shortening of AERP and increased vulnerability for AF by single premature stimuli.28-29 Chronic atrial stretch and/or enlargement on the other hand (as in patients with ASD), have consistently been associated with a significant increase in AERP and susceptibility to AF with single extrastimulus. A number of studies have shown that significant (at least 40%, by some)30 atrial enlargement is associated with AERP prolongation, increased p-wave duration and extended sinus node recovery time (SNRT).31-35 Biatrial enlargement due to AV dysynchrony by long-term VVI pacing has similarly been associated with prolongation of the AERP36-37
Electrical remodeling due to atrial enlargement contrasts the type of remodeling observed after RAP or AF. For the first time in an animal model, Wijffels et al38 discovered that (artificially-induced and maintained) AF produced significant electrophysiologic changes in the atrial myocytes, namely a decrease in AERP, which caused AF to become sustained and unable to terminate spontaneously (hence, “AF begets AF”).38 Subsequent studies have confirmed the association of AF (and RAP) with marked decrease in AERP, as well as with significantly increased atrial impulse wavelength, loss of rate adaptation of the refractory period, sinus node dysfunction and accelerated conduction velocity, thus supporting the clinical experience that the success rate of chemical or electrical cardioversion of AF is higher when AF has lasted only a short time.39-51
Changes in atrial refractoriness has been explained at the atrial myocyte level by the concept of “ionic current remodeling” in the presence of congestive heart failure (CHF) and RAP. More specifically, with chronic atrial enlargement, there is a modest decrease in both L-type Ca2+ currents and transient outward (Ito) and slow delayed-rectifier (IKs) K+-currents, no change in inward rectifier, ultrarapid and rapid delayed rectifier, and T-type Ca2+ currents, and an increase in the Na+, Ca2+-exchanger (NCX) current. The net result is an increase in action potential duration (APD) at faster rates, paralleling AERP prolongation noted in vivo.52-53 RAP on the other hand, has been associated with a significant reduction in the densities of Ito and Ica with subsequent reductions in APD and APD adaptation to rate and shorter atrial refractoriness.54-56
While shortening of the AERP has clearly been shown to predispose and sustain AF, lengthening of the AERP (as present in ASD patients with chronic atrial enlargement) may still increase atrial susceptibility to fibrillate by a different mechanism. Atrial refractoriness appears to be heterogeneously dispersed across key anatomic areas of the right and left atrium, most notably the crista terminalis (CT) and the posterior wall of the left atrium (PLA), respectively.57 Ultrastructural analysis of the CT reveals a simple end-to-end communication between the myocytes with numerous gap junctions in intercalated disks and minimal connections between laterally apposed cells, hence the propagation velocity in the CT is approximately 10 times greater in the longitudinal than in the transverse direction leading to anisotropic conduction preferentially along the anatomic LoB.58 The increase in atrial refractoriness observed with atrial dilatation is unequally distributed across the RA (anisotropic dispersion of AERP), with AERP significantly prolonged along the thin free wall compared to the thick CT (functional LoB).32-33 The combination of both anatomical and functional LoB’s predisposes the RA to re-entrant tachyarrhythmias presenting as atrial flutter or atrial fibrillation, depending on the length of the overall LoB. A shorter LoB is more likely to “short-circuit” and become unstable, degenerating into AF. RAP and spontaneous atrial tachyarrhythmias have already been shown to produce a functional LoB between the vena cavae and its length was found to be critical for the maintenance of AFL and in preventing its degradation into AF.59-61 The PLA is another key site where a line of anatomic LoB can be drawn perpendicularly between the superior and inferior pulmonary veins, correlating well with a region of change in subendocardial fiber orientation. This line is responsible for the breakup of wave fronts into multiple wavelets during the initiation of AF from the pulmonary vein foci.62 In the presence of chronic LA dilation, conduction along the anatomic LoB of the PLA becomes more anisotropic due to heterogeneous dispersion of atrial refractoriness (functional LoB) creating a favorable substrate for re-entry and fibrillatory activity.57,63
However, even after prolonged periods of AF (months or years), electrical remodeling appears to be completely reversible and atrial refractoriness becomes normal within a few days of sinus rhythm. Allessie et al46 suggested that a “second factor” was responsible for persistent AF. Good candidates included “atrial stunnig” (loss of atrial contractility, contractile remodeling) and atrial interstitial fibrosis (structural remodeling). Down-regulation of ICaL, pronounced reduction of Ca2+ -transient current and Ca overload intracellularly were shown experimentally to cause atrial stunning.55,64-65 The duration of contractile dysfunction correlated well with the duration of AF and could take months prior to full recovery.66 Still, atrial fibrosis is the hallmark of AF “domestication” by maintaining an arrhythmogenic substrate. Atrial fibrosis is the convergent pathologic end point of a variety of cardiac insults, including chronic atrial stretching (such as with an ASD), and a reparative process to replace degenerating myocardial cells.67-68 Mechanical stretch induces increased angiotensin II (AngII) and transforming growth factor (TGF) –beta 1 expression and collagen synthesis in cardiac fibroblasts. It also stimulates cardiomyocyte signaling via activation of the angiotensin II type 1 (AT1) receptors and mitogen-activated protein kinase, with direct fibroblast-activating effects.69-73 A lower percentage of atrial cardiomyocytes (compared to the ventricles, 45% vs 76%), and thus, a larger concentration of fibroblasts, inevitably leads to pronounced atrial extracellular matrix accumulation and remodeling.74-75 Fibrosis has significant electrophysiologic effects, namely, it creates localized areas of muscle fiber disarray and thinning causing conduction slowing, increased conduction heterogeneity, unidirectional conduction blocks and smaller and more numerous reentrant circuits, a necessary substrate for AF.76-80 Atrial myocytes, on the other hand, assume a more fetal phenotype (dedifferentiation), which resembles the changes in ventricular myocytes due to chronic ischemia (hibernation); such changes are initially reversible and heterogeneously distributed, however, in dilated atria (especially the RA), progression to DNA cleavage, programmed cell death and replacement with fibrous tissue, have been observed.46,81
Therefore, patients with significant ASDs should be offered elective closure once the diagnosis is made. Current indications for ASD closure include right atrial and ventricular dilation by echocardiography, MRI or CT associated with ASD minimum diameter > 10mm on echocardiography and/or Qp:Qs > 1.5:1.0 by echocardiographic or cardiac MRI flow assessment or from oxygen saturation runs, when cardiac catheterization is performed. Treatment includes surgical repair of the ostium primum and sinus venosus ASDs, as well as of the ostium secundum ASDs, which are unsuitable for percutaneous device closure (defect > 36mm, inadequate atrial septal rims for stable device deployment, or proximity of the defect to the AV valves, the coronary sinus or the vena cavae).19,82 Mortality and symptoms are significantly improved after surgical repair of ASDs, regardless of age, pulmonary arterial pressure (PAP) or pre-operative functional class.22,83-84 On the other hand, the incidence of atrial tachyarrhythmias (most notably AF and AFL) is significantly age-dependent, with a higher incidence of AF and AFL among patients undergoing surgical closure in adulthood. Multiple studies have shown that preoperative, persistent and new-onset (i.e. postsurgical) AF or AFL are significantly more common among older patients.21,23-25,83,85-92 For instance, Gosh et al observed a lower incidence of preoperative AF (23.5% vs 43.6%, p < 0.05) among patients 35-50 years old than among patients older than 50 years and a lower incidence of new AF (7.8% vs 34%, p < 0.05) in the former than the latter group.93 The reported incidence of AF and AFL after surgical closure of ASD at adult age was indeed comparable to the incidence of these arrhythmias in natural history studies of ASD patients, namely, 15-40% in 30-35 year-olds.7,18 Some studies even claimed a higher incidence of new onset supraventricular arrhythmias in surgical patients than in patients receiving medical management (39% vs 14%, p=0.01).89,94 These numbers are significantly different from the 3% incidence reported by Roos et al for ASD patients operated in childhood.95 The etiology of persistent or new-onset atrial arrhythmias following surgical repair of ASD in adults in not completely understood, however, the extent of residual RA dilatation after ASD closure appears to play a critical role. Indexed RA area remains increased after ASD repairs in adults compared to that in control subjects (still relatively decreased over time) and the decrease in RA area is inversely proportional to the patient’s age at the time of the ASD closure.96 As shown earlier, persistent atrial dilatation is essential for the initiation and domestication of AF and AFL. In addition, surgical scars (from the ASD repair) in the RA free wall act as anatomical LoB allowing either re-entry phenomena and AFL or scattered atrial electrical activity and AF to take place depending on the length of the LoB.59-61,97-99 Finally, parameters such as the use of cardioplegia, type of cannulation, location of the defect and the presence of abnormal pulmonary venous drainage have been associated with sinus node injury or dysfunction and atrial arrhythmias.21-24 The unacceptably high rates of persistent or new AF and AFL after adult ASD surgery (with their associated risks of stroke and chronic drug therapy) in conjunction to the small added risk of performing an AF surgical procedure, such as the modified Cox-Maze procedure, support their combination in such high risk patients.100-101 The combination of the two surgical procedures has been associated with an approximately 85% rate of restoring sinus rhythm, a 90% rate of maintaining sinus rhythm and a significant improvement in New York Heart Association classification 8 months after combined surgery102-103 with rates heavily dependent on the pre-operative size of the left atrium and the magnitude of the atrial fibrillatory wave103
Nevertheless, surgical repair of ASDs carries significant morbidity and mortality.23,89,99,104 Percutaneous closure has recently emerged as an attractive alternative to the traditional open-chest procedures. The new modality has been associated with significant hemodynamic and electrophysiologic improvements in cardiac function. RV volumes are significantly decreased in < 24 hours after the procedure and continue to improve for up to 6 months (-not observed with surgical ASD repair due to the detrimental effects of cardioplegia).108-110 Improvement in RV filling pressures and function after percutaneous ASD repair have been associated (via the principle of ventricular interdependence) with an improved LV diastolic function, as suggested by normalization of the LV isovolumic relaxation time and LV end-diastolic dimension, and systolic function, as suggested by a statistically significant increase in LV ejection fraction.84,109,111-112 Improved ventricular filling and atrial preload parameters may lead to noticeable reductions in indexed RA and LA areas (though, without complete resolution) over time in an age-dependent fashion.96,106,113 Early percutaneous repair of ASDs has been associated with a decreased incidence of atrial tachyarrhythmias and ectopy compared to surgical closure.105,114
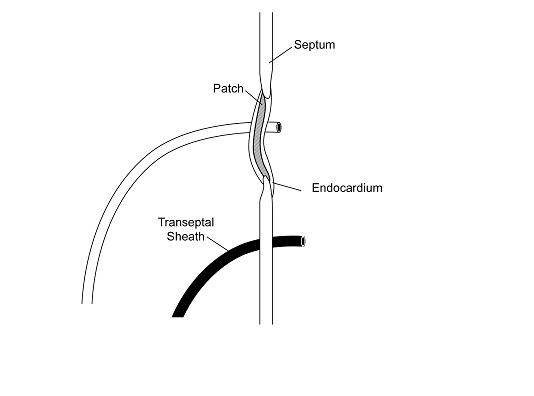
At any rate, patients who develop AF after ASD repair or PFO closure maintain the option of AF ablation irrespective of the closure (surgical or percutaneous) modality chosen. Concerns regarding gaining access to the LA via a transeptal puncture (TSP) under fluoroscopic guidance in the presence of an intra-atrial patch closure device initially led to reasonable hesitations in percutaneously treating AF. Alterations in the septal anatomy after repair and the potential for mechanical dislodgement and damage to the closure apparatus made the complexity of the procedure, risks of complications and the fluoroscopy time prohibitive to many operators. Nevertheless, a few studies have shown that TSP under these conditions can be performed with low risk of complications. In a study of 39 pediatric patients with congenital heart disease requiring intra-atrial patch (IAP), the IAP was successfully punctured in 97.5% of the patients (38/39) with no complications; follow-up echocardiography in 79% of the patients revealed no residual shunting across the atrial septum except for two cases of atrial baffle, which were intentionally fenestrated.115 Double TSP for AF ablation was successfully (95%) performed under the guidance of intracardiac echocardiography (ICE) in a prospective study of 20 post-ASD/PFO repair patients, age- and gender-matched with 20 control patients. Lakkireddy et al showed that surgical repairs with synthetic materials like Dacron (but not Gore-Tex) can safely be punctured to gain access to the LA, while most ASD or PFO closure devices typically occlude the anteroseptal portion of the interatrial septum, leaving a relatively wide posteroinferior area for TSP figures 1-2. No acute or long-term complications were noted and echocardiographic follow-up at 3 months showed residual interatrial communication in only 1 patient, which resolved in 12 months.116
Figure 1. Cross section interatrial septum showing the transseptal sheath inferior and posterior to the closure device (courtesy of Lakkireddy D, Patel D, Mlcochova H, Thal S, Wazni O, Kanj M, Arruda M, Prasad S, Bhargava M, Cummings J. Feasibility of transeptal puncture in radiofrequency catheter ablation of atrial fibrillation in patients with atrial septal defect (ASD) repair. Heart Rhythm. 2006; 3(5):s282-3 [Abstract])
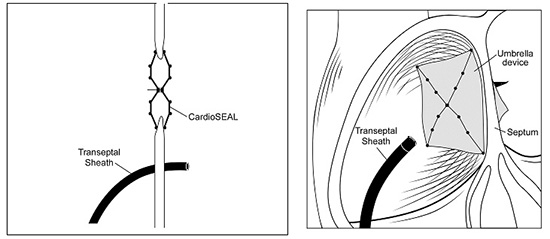
Figure 2. IInteratrial septum with surgical closure patch through which a transseptal sheath was placed. Note an additional sheath that was placed at a more posterior and inferior location. (courtesy of Lakkireddy D, Patel D, Mlcochova H, Thal S, Wazni O, Kanj M, Arruda M, Prasad S, Bhargava M, Cummings J. Feasibility of transeptal puncture in radiofrequency catheter ablation of atrial fibrillation in patients with atrial septal defect (ASD) repair. Heart Rhythm. 2006; 3(5):s282-3 [Abstract])
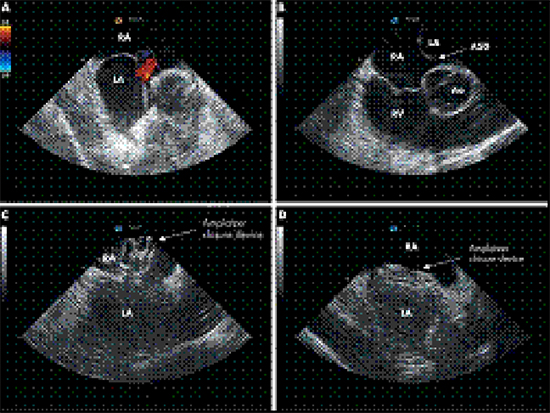
Overall, percutaneous ASD closure is a safe and efficient procedure with only rare (less than 1%) serious complications in experienced centers. Case reports of device failure to deploy, malfunction, fracture or embolization, myocardial perforation and hemopericardium (with or without tamponade), residual shunts, thrombus formation on the device and aortic sinus-to-left atrial fistula formation have been reported.117-122 In an effort to improve the safety and success of percutaneous (and surgical) ASD procedures, cardiac imaging such as transesophageal echocardiography (TEE) and ICE have been incorporated in all ASD closure procedures. TEE has been helpful in the selection of patients for surgical or percutaneous repair, in assessing the morphology, dimensions and relationship of ASD to the other intracardiac structures, in guiding the operator during the procedure and in early detection of any ensuing complications. Although the risks of TEE are minimal (< 0.02%), insertion of the TEE probe can potentially result in oral, esophageal or pharyngeal trauma, arrhythmias and patient discomfort. General anesthesia is usually required and additional staff (anesthesiologist, TEE operator) need to be present in the lab throughout the procedure. Experienced TEE operators are crucial for acquisition of quality images and reducing procedure and fluoroscopy times.123-125 ICE has emerged as the preferred alternative to TEE. It provides accurate visualization of the intracardiac anatomic structures, defect(s) and closure devices or patches and guides the operator in interventions, such as TSPs and placement of closure devices, thus preventing complications associated with the procedure Figure 3 . It also helps in microbubble monitoring for power adjustment during radiofrequency ablation and in visualization of thrombus formation on catheters and sheaths.126-132 In contrast to TEE though, it avoids esophageal probe insertion (and its associated risks), patient discomfort and general anesthesia.
Figure 3. Closure of an atrial septal defect (ASD) under ICE guidance. (A) Colour Doppler demonstrating an ASD located in the inferior part of the atrial septum. (B) Relation of the ASD (arrow) to intracardiac structures. (C) Positioning of the Amplatzer closure device. (D) After several minutes, flattening of the closure device occurs. The device is positioned stable in between the right and left atria. Ao, aorta; LA, left atrium; RA, right atrium; RV, right ventricle
Medical Management of AF in ASD Patients
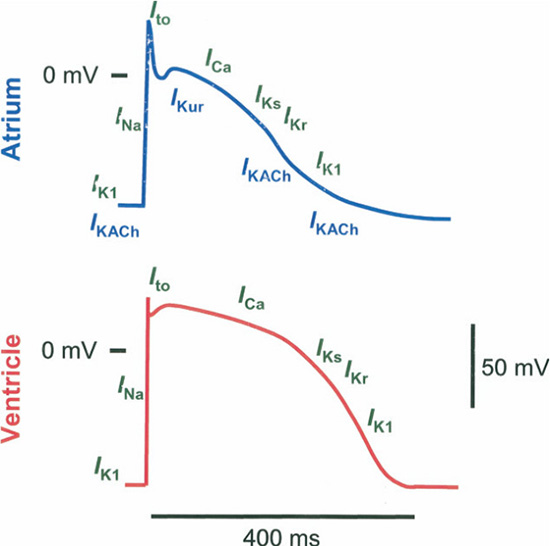
Since AF is so common in the group of ASD (and especially, the unrepaired or adult-repaired ASD) patients, medical management remains essential. Pharmacologic agents to convert AF and prevent relapses have traditionally included antiarrhythmic drugs of the Vaughan-Williams class I and III. However, these agents are notorious for their proarrhythmic effects and poor tolerability. Latest breakthroughs in the understanding of the pathophysiologic mechanisms behind the initiation and maintenance of AF (and other atrial tachyarrhythmias) have inspired rigorous interest in the pharmacologic targeting of the various steps. The concept of electrical remodeling in AF has led research efforts towards “atrial-selective” drugs, that is, modulators targeting ion-specific-channels Figure 4 (most notably Ikur133 and IkAch),134 stretch-activated nonselective cation channels (SACs)135 and atrial structures (such as atrially expressed connexins),136 to affect atrial electrophysiologic activity.
Figure 4. Regional Difference in Ionic Current Contribution to APs. Differences in key ion currents between atrial (blue) and ventricular (red) action potentials (APs). Atrial APs have smaller AP amplitude and less negative resting membrane potential. Currents (green) identified above the line illustrating the APs are present in both regions; atrially expressed ionic currents are illustrated below the AP schematic (blue). IKACh _ acetylcholine-regulated potassium current; IKur _ ultrarapid delayed-rectifier potassium current; INa _sodium current (courtesy of Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J Am Coll Cardiol. 2008 Feb 26;51(8):787-92)
Most of the currently investigated modulators though are not in strict terms “specific”, but rather one or two orders of magnitude more selective in inhibiting the target(s) of interest compared to other potential targets.137 Structural remodeling due to interstitial fibrosing is another potential target of pharmacotherapy and important feature of AF substrate. Several profibrotic factors have been discovered, most notably, AngII, TGF-b1, PDGF and connective tissue growth factor, and the renin – angiotensin – aldosterone pathway has extensively been implicated in the process.73 Recent therapeutic agents such as ACE inhibitors, angiotensin II type 1 receptor blockers and antialdosterone therapies specifically target the latter and have been shown in multiple studies to substantially reduce fibroting changes and AF.140-141 Similarly, 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase inhibitors142-143 and omega-3 polyunsaturated fatty acids were found to delay tissue fibrosis and thus prevent AF.144 Gene therapy remains the last frontier in AF therapy with the most promise for atrial selectivity but is still lacking clinical applications.
In conclusion, AF in ASD patients is mainly the byproduct of RA and LA dilation. Changes in atrial refractoriness, ionic currents and atrial conduction properties (electrical remodeling), along with tissue remodeling due to atrial fibrosis (structural remodeling) generate a favorable substrate for the initiation and domestication of AF (and other atrial tachyarrhythmias). Surgical repair of ASD remains the standard therapeutic modality except for a selective population of patients, for which a percutaneous approach is preferred. Adult repair is associated with similar rates of AF in comparison to unrepaired ASD, partly due to residual RA dilation with fibrotic changes. Current AF treatment in ASD patients includes the modified Cox-Maze procedure (usually concomitantly with the ASD repair), percutaneous AF ablation via TSP and pharmacologic modalities, currently focusing on atrially expressed targets and pathophysiologic steps in atrial remodeling. Genetic manipulation is a promising research field in AF.