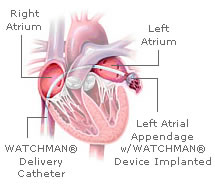
Stroke is a major risk factor for patients with Atrial Fibrillation (AF). Treatment therefore revolves around anticoagulation to prevent thrombus formation and dislodgement from the left atrial appendage. Warfarin is used for anti-coagulation but is associated with several disadvantages. It has a narrow therapeutic window and is also associated with bleeding complications. An alternate treatment strategy includes occlusion of the left atrial appendage. Recently, FDA has reviewed an application submitted by the Aritech company for a left atrial appendage (LAA) closure Device. This Watchmen LAA closure device is a self expanding nickel-titanium alloy that can be introduced percutaneously via the femoral access and placed in the LAA.
The device was studied in a multi center prospective, unblinded pivotal clinical trial called PROTECT- AF (protection in patients with Atrial Fibrillation). Participants included patients with non-valvular atrial fibrillation who were deemed eligible for coumadin therapy. Patients were randomly assigned to either receive anticoagulation with coumadin or the watchman closure device with 45 days of coumadin. Primary end point was a composite of absence of death, embolism and stoke (ischemic and hemorrhagic).
The results show a 32% reduction of these events in the LAA closure device group after 900 patent years of observation. These results should however be interpreted with caution! The implantation of the device is associated with 12.3% procedural risks while the success rate of implantation is 90.9%. Complications included serious pericardial effusions which required drainage or surgery, acute ischemic stroke from air or thromboembolism and even post implantation sepsis or device embolization. There is also a learning curve associated with the procedure and less experienced operators had higher rates of pericardial effusion. Furthermore, the device implantation does not preclude anticoagulation, and patients receive anti-coagulation or antiplatelet therapy or both for 45 days to prevent thrombus formation on the surface.
As per the PROTECT-AF protocol, anticoagulation was withdrawn after 45 days if transesophageal echo showed complete occlusion of the LAA. While 90% of patients could discontinue warfarin within 60 days, 10% had to restart anti-coagulation for several reasons including thrombus on the device despite heparinization at implantation, 45 days of warfarin and anti-platelet therapy. The rate of ischemic stroke was also higher (3% vs 2%) in the device group than the anticoagulation group. While these are ischemic events, rates of subclinical cerebral infarcts could be higher.
PROTECT-AF had a small sample size of less than 100 patients and thus the efficacy estimate is not precise. Inclusion criteria for the study was patients eligible for warfarin therapy and thus included patients with a CHADS2 score ranging from 1 to 6. 30% of patients in the watchman device group only had a CHADS2 score of 1 which meant these patients only required aspirin and not coumadin. On the other hand, the device could be most useful in patients who are at high risk for bleeding in whom warfarin cannot be used. But these patients were excluded from the study. As device implantation requires aggressive peri-procedural anticoagulation, the risk and benefit in these patients is uncertain. Furthermore, 25% of the strokes in patients with AF may occur due to intrinsic cerebrovascular disease or emboli from atheromatous aorta and not from the LAA thrombus.
Providing long term anticoagulation to patients with AF is challenging and LAA closure device offers an alternative. This device can be a promising option for select candidates and routine implantation may not be warranted at this time.
 |
 |
|
(source: atritech.com) |
|
Display Ads on JAFIB to reach Users world wide...
Suggest an Event to get good mileage from Users World wide...