Pulmonary Vein Remodeling Following Atrial Fibrillation Ablation: Implications For The Radiographic Diagnosis Of Pulmonary Vein Stenosis
Faisal M. Merchant, MD1, Mathew R. Levy, BS1, Shahriar Iravanian, MD1, Ramal M. Weragoda, MD1, Edward C. Clermont, MD2, Heval M. Kelli, MD3, Robert L. Eisner, PhD4, David Vadnais, MD3, Mikhael F. El-Chami, MD1, Angel R. Leon, MD1, David B. Delurgio, MD1
1Cardiology Division (Section of Cardiac Electrophysiology), Department of Medicine, Emory University School of Medicine, Atlanta, GA.2Cardiology Division, Department of Medicine, University of Pennsylvania, Philadelphia, PA.3Cardiology Division, Department of Medicine, Emory University School of Medicine, Atlanta, GA.4Department of Radiology, Emory University School of Medicine, Atlanta, GA.
Pulmonary vein (PV) reverse remodeling has been recognized following atrial fibrillation (AF) ablation. However, the extent of physiologic reverse remodeling after AF ablation and the potential impact of reverse remodeling on the radiographic diagnosis of PV stenosis have not been well characterized.
From January 2004 to February 2014, 186 patients underwent paired cardiac magnetic resonance imaging (MRI) to delineate PV orifice dimensions before and after (mean 109 ± 61 days) an initial AF ablation.
Negative remodeling of the PV orifice cross sectional area occurred in 67.8% of veins with a mean reduction in area of 21.0 ± 14.1%, and positive remodeling was seen in the remaining PVs with an increase in area of 22.1 ± 23.4% compared to baseline. No PVs demonstrated a reduction in cross-sectional area of > 75% (maximum reduction observed was 58%). Negative remodeling of the PV long axis dimension was observed in 55.2% of veins with a mean reduction of 14.6 ± 9.2% compared to pre-ablation and positive remodeling was observed in 25.3% of PVs with a mean increase in diameter of 14.7 ± 12.6%. Only 1 PV demonstrated a reduction in orifice diameter of > 50%. There were no clinically evident or suspected cases of PV stenosis in this cohort.
Negative remodeling of the PV orifice area was noted in the majority of PVs following AF ablation. However, in almost all cases, the extent of negative remodeling was well below commonly used thresholds for the radiographic diagnosis of PV stenosis.
Key Words : Atrial Fibrillation, Ablation, Pulmonary Veins, Pulmonary Vein Stenosis, Cardiac Magnetic Resonance.
Correspondence to: Faisal M. Merchant, Emory University Hospital Midtown, 550 Peachtree Street, MOT 6th floor, Atlanta, GA 30308.
Pulmonary vein stenosis (PVS) is an important complication of catheter ablation for atrial fibrillation (AF). Although the incidence of PVS has decreased with the evolution from ostial to more antral PV isolation and other advances in ablation technology, a recent systematic review suggested that the incidence of PVS in contemporary studies remains approximately 2%.1 In the Second Worldwide Survey of AF ablation, the incidence of PVS requiring invasive treatment was 0.29% among patients undergoing ablation from 2003 to 2006;2 however, this figure is likely subject to underreporting, particularly given that most operators do not routinely screen asymptomatic patients for PVS following ablation.3 The diagnosis of PVS depends on a combination of symptoms, cross-sectional imaging such as computed tomographic (CT) or magnetic resonance (MR) angiography, functional assessment such as lung perfusion scanning and findings at diagnostic catheterization including pulmonary venography and hemodynamic assessment of stenosis severity. In patients with symptoms concerning for PVS following AF ablation, cross-sectional imaging is frequently used as an initial diagnostic step and helps guide further evaluation. Commonly cited radiographic criteria for the diagnosis of PVS include a reduction in PV long axis diameter of >50% compared to pre-ablation1,4 or a reduction in PV cross-sectional area of >75% (corresponding to a reduction in diameter of ~50%).5 These thresholds were defined primarily based on early series of patients presenting for evaluation of clinically suspected PVS where it was noted that symptoms typically emerged when the severity of stenosis reached a threshold of approximately 60% reduction in diameter.6,7 However, it is well recognized that AF ablation and maintenance of sinus rhythm may result in reductions of left atrial (LA) size and pressure, with concomitant reverse remodeling of the PVs and a physiologic reduction in PV size.8-14 However, the incidence and extent of physiologic reverse remodeling associated with AF ablation and the manner in which reverse remodeling may impact the radiographic diagnosis of PVS have not been well described.
The Emory University institutional review board approved the study protocol. Patients at Emory University Hospital Midtown undergoing initial catheter ablation for atrial fibrillation between January 2004 and February 2014 who had paired pre- and post-procedure cardiac magnetic resonance (CMR) imaging performed to delineate PV anatomy were included in this analysis. Post-procedure CMR was performed in all patients in this cohort to screen for PVS. Baseline demographic data, clinical covariates and procedural details were ascertained by review of electronic medical records. The decision to perform AF ablation along with specific details of the ablation strategy and peri-procedural management were performed at the discretion of the treating physician.
Pre- and post-ablation gadolinium-enhanced CMR scans were performed on a 1.5 tesla Philips Intera® MRI scanner (Amsterdam, Netherlands) using a 5-element phased-array cardiac coil. Turbospin echo and gradient echo images in axial and double oblique planes following the administration of gadopentetate dimeglumine (Magnevist®) or gadobenate dimeglumine (MultiHance®) (dosed at 0.075-0.10 mmol/kg) were used to delineate PV anatomy. Orthogonal projections of angiographic images were used to measure PV dimensions (Figure 1). PV orifice cross-sectional area was calculated from the ostial long and short axis dimensions assuming an ellipsoid shape.15
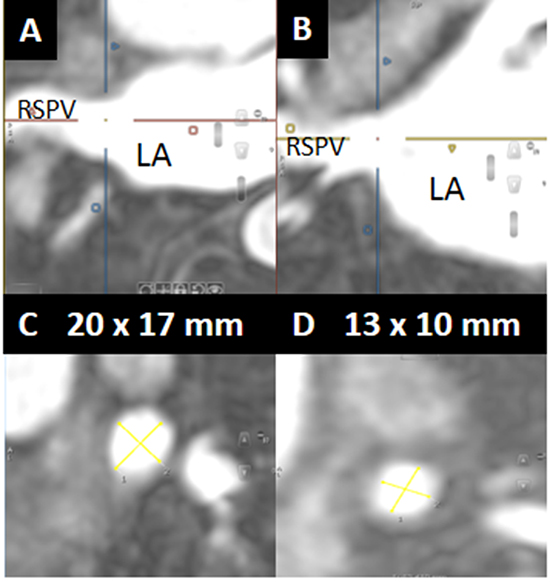
Figure 1. A and B. Example of orthogonal projections on cardiac magnetic resonance imaging used to define the pulmonary vein (PV) orifice. C and D. Long and short axis measurements of the PV orifice used to calculate the orifice cross-sectional area. Pre-ablation (panel C), the right superior PV measured 20 x 17 mm. Post-ablation (panel D), it measured 13 x 10 mm. RSPV = right superior pulmonary vein, LA = left atrium.
From the 186 patients in this cohort, 18 patients (9.7%) had a left common PV and in these cases, the left-sided veins were excluded from the analysis of PV remodeling, resulting in a total of 708 individual PV orifices for analysis.
Continuous variables are presented as mean ± standard deviation and categorical data are summarized as frequencies and percentages. Comparisons across groups were performed using the Student’s T-test or Fisher’s exact test, as appropriate. Correlations between continuous variables were performed using Pearson correlation coefficients. For all comparisons, a two-tailed p < 0.05 was considered to be statistically significant. Analysis was performed using STATISTICA software (Statsoft, Inc., Tulsa, OK).
During the period of interest, 186 patients underwent paired pre- and post-procedure CMR scans associated with a first AF ablation procedure. Mean age of patients in this cohort was 58.3 ± 9.8 years, 82% were male and 24% had persistent AF. Other baseline characteristics are presented in Table 1. The vast majority of patients in this cohort underwent antral PV isolation with radiofrequency (RF) (n=181) and 5 patients underwent Cryoballoon ablation. There were no significant differences in baseline characteristics between patients in the 2 ablation modality groups. The post-procedure CMR was performed 109 ± 61 days following ablation. No patients in this cohort had clinically evident or suspected PV stenosis.
Table 1. Baseline characteristics
|
n = 186 |
| Age (years) |
58.3 ± 9.8 |
| Male gender |
152 (81.7) |
| Hypertension |
76 (40.9) |
| Coronary artery disease |
14 (7.5) |
| Diabetes mellitus |
7 (3.8) |
| Obstructive sleep apnea |
20 (10.8) |
| Persistent atrial fibrillation |
45 (24.2) |
| Medical therapy at the time of ablation |
|
| Beta blockers |
57 (30.6) |
| Non-dihydropyridine calcium channel blockers |
18 (9.7) |
| Angiotensin antagonists |
37 (19.9) |
| Statins |
47 (25.3) |
| Warfarin |
80 (43) |
| Novel oral anticoagulants |
24 (12.9) |
| Amiodarone |
12 (6.5) |
| Dronaderone |
17 (9.1) |
| Sotalol |
32 (17.2) |
| Dofetilide |
9 (4.8) |
| Class Ic antiarrhythmics |
55 (29.6) |
Data are presented as mean ± standard deviation or n (%)
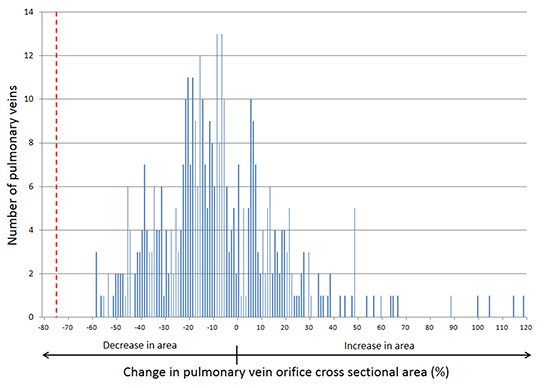
At baseline (prior to ablation), the mean cross-sectional areas of the four PVs were: left superior PV (LSPV) 202.6 ± 66.6 mm2; left inferior PV (LIPV) 177.9 ± 84.4 mm2; right superior PV (RSPV) 268.4 ± 114.9 mm2 and right inferior PV (RIPV) 221.9 ± 86.6 mm2. At the time of follow-up CMR, negative remodeling of the PV orifice (defined by a reduction in cross sectional area) was observed in 67.8% of veins, with a mean reduction in area of 21.0 ± 14.1% and positive remodeling was seen in the remaining PVs with an increase in area of 22.1 ± 23.4%. Averaging all veins, the mean percent change in PV orifice cross sectional area for each of the four PVs ranged from a mean reduction of 5.1% in the RIPV to a reduction of 9.8% in the RSPV (Table 2). For all PVs, the mean change in cross sectional area is plotted in Figure 2 and approximates a bell-shaped distribution, with the peak centered in the range of roughly 5-10% reduction in orifice area across all veins. The largest decrease in orifice area in any vein observed was 58%, well below the currently used threshold of 75% for the radiographic diagnosis of PVS (marked by the dashed red line in Figure 2).
Figure 2. Distribution of changes in pulmonary vein orifice cross sectional area observed after atrial fibrillation ablation. The dashed red line marks the 75% reduction in cross sectional area threshold which is commonly used for the radiographic diagnosis of pulmonary vein stenosis
There were some statistically significant, although relatively weak correlations in the extent of negative or positive remodeling between PVs. The strongest correlation was between the LSPV and RIPV (r = 0.247, p = 0.02). Other significant correlations included the LSPV vs. LIPV (r = 0.239, p = 0.03) and LSPV vs. RSPV (r = 0.220, p = 0.04). Other correlations were not significant. At the patient level, out of 186 patients included in this cohort, 44 demonstrated evidence of concordant positive remodeling, meaning that all PVs showed an increase in orifice size following ablation whereas only 5 patients showed evidence of concordant negative remodeling. This data highlights that although over two-thirds of PVs demonstrated negative remodeling, in the vast majority of patients, at least one PV showed an increase in size, leading to lack of concordance in negative remodeling. In contrast, when positive remodeling was present, it was much more likely that all PVs increased in size leading to a concordant pattern. No significant difference was noted in the need for cardioversion in the first 3 months following ablation among patients with, compared to those without, concordant positive remodeling.
Table 2. Changes in pulmonary vein orifice size
|
Baseline cross sectional area (mm2) |
Mean change in cross sectional area (%) |
Percentage of patients with change following ablation |
| Reduction |
No change |
Increase |
| Left superior pulmonary vein |
202.6 ± 66.6 |
-6.4 ± 26.5 |
61.8 |
|
38.2 |
| Left inferior pulmonary vein |
177.9 ± 84.4 |
-7.7 ± 28.5 |
73.4 |
|
26.6 |
| Right superior pulmonary vein |
268.4 ± 114.9 |
-9.8 ± 24.5 |
69.8 |
|
30.2 |
| Right inferior pulmonary vein |
221.9 ± 86.6 |
-5.1 ± 26.8 |
67.5 |
|
32.5 |
|
Baseline long axis diameter (mm) |
Mean change in diameter (%) |
Percentage of patients with change following ablation |
| Reduction |
No change |
Increase |
| Left superior pulmonary vein |
18.0 ± 3.3 |
-5.1 ± 14.6 |
56.5 |
20.9 |
22.6 |
| Left inferior pulmonary vein |
17.5 ± 3.3 |
-6.0 ± 16.3 |
63.1 |
22.3 |
14.6 |
| Right superior pulmonary vein |
20.3 ± 4.3 |
-5.2 ± 15.4 |
53.9 |
20.6 |
25.5 |
| Right inferior pulmonary vein |
18.3 ± 3.7 |
-2.0 ± 13.4 |
49.3 |
21.1 |
29.6 |
Data are presented as mean ± standard
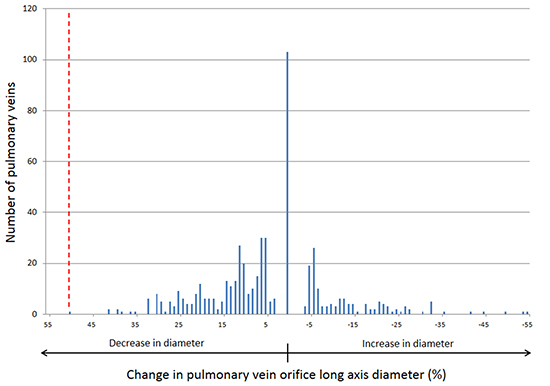
Data on PV long axis diameter are also presented in Table 2. Following ablation, approximately half of PVs demonstrated a reduction in diameter, about a quarter increased in diameter and no change was noted in the remaining patients. The distribution of changes in PV diameter following ablation is plotted in Figure 3. The maximum observed decrease in PV diameter following ablation was 51%, which is just above the commonly used threshold of 50% (marked by the dashed red line in Figure 3). With the exception of that single PV, all others demonstrated changes in diameter well below the 50% threshold.
Figure 3. Distribution of changes in pulmonary vein orifice diameter observed after atrial fibrillation ablation. The dashed red line marks the 50% reduction in diameter threshold which is commonly used for the radiographic diagnosis of pulmonary vein stenosis
Our data demonstrate that AF ablation is associated with negative remodeling of the PV orifice area in about two-thirds of PVs, and the expected physiologic change in PV orifice area follows a roughly bell-shaped distribution with a mean reduction in orifice area of about 5-10%. In about one-third of PVs, positive remodeling is observed after ablation and when present, positive remodeling is more likely to affect all PVs in a concordant pattern. Importantly, the physiologic range of negative remodeling observed is well below the thresholds currently used for the radiographic diagnosis of PV stenosis.
Numerous prior studies have assessed the extent of PV negative remodeling associated with AF ablation by comparing paired baseline and follow-up imaging studies (both CT and MR), usually performed at a mean follow-up of approximately 2 - 4 months post-ablation. In these studies, the mean reduction in PV diameter following ablation has ranged from 6.5 - 16%.10,11,13,16 In similarly designed studies, it has been noted that the percentage of PVs that can be expected to demonstrate evidence of negative remodeling ranges from 22 - 38%.9,11,14 Our data add to this existing body of literature in several ways. First, a major difference between prior studies assessing PV remodeling after AF ablation and our analysis is that most prior studies have focused on a single PV diameter measurement rather than assessing cross-sectional area. However, it is well recognized that the PV orifice in most patients is ellipsoid, not spherical15,17,18 and therefore, a single long-axis diameter measurement does not fully represent the orifice size or the degree of hemodynamic impact from narrowing of the PV orifice. When assessing cross-sectional area, our data suggest that nearly two-thirds of PVs demonstrate evidence of negative remodeling after ablation. In contrast, prior studies looking only at changes in diameter, but not in area, have suggested that the percentage of PVs that demonstrate negative remodeling is lower, ranging from 22 – 38%.9,11,14 Additionally, whereas most previous studies have focused on the extent of negative remodeling, our data also incorporates the extent of positive remodeling and in doing so, we are able to demonstrate that the physiologic range of PV orifice remodeling following ablation approximates a bell-shaped distribution, with a mean change in cross-sectional area of around negative 5-10% (Figure 2).
Having demonstrated such a bell-shaped distribution, it is interesting to speculate on the potential mechanisms for negative and positive remodeling. Although the pathophysiologic mechanisms which lead to PVS have not been precisely defined, it has been speculated based on histologic studies that inflammatory injury leads to collagen deposition, intimal proliferation, fibrosis and eventually endovascular contraction.1 However, it seems probable that the mechanisms leading to physiologic negative remodeling are likely distinct from the patterns of injury that lead to clinically relevant PVS. It has been speculated that successful catheter ablation, and the maintenance of sinus rhythm, may lead to reductions in LA volume and pressure, with a corresponding reduction in PV size. Although some studies have demonstrated a correlation between post-ablation reduction in LA volume and PV size13 others have suggested that the magnitude of PV remodeling differs between PVs and does not necessarily correspond with changes in LA volume.12,16 Our data are more consistent with the later in demonstrating that correlations between PVs in the extent of remodeling were relatively weak and although the majority of PVs demonstrated evidence of negative remodeling, within individual patients, there was significant variability in the pattern of PV remodeling leading to a lack of concordance in negative remodeling. The correlation between maintenance of sinus rhythm after ablation, LA pressure and PV remodeling is likely complex and requires further elucidation.
Defining the expected range of physiologic remodeling after AF ablation has important relevance for the diagnosis of PVS. Although PVS has become less common with advances in ablation technology and evolution from ostial to antral lesions, a recent systematic review suggested that the incidence of PVS in contemporary studies ranges from 3-8% (mean ~2%)1 and these figures may be an underestimate since most operators do not screen for PVS in the absence of clinical concern.3 As such, determining the risk of PVS remains an important step in defining the safety of new ablation technologies. For instance, in a recent analysis of 50 patients undergoing ablation with the pulmonary vein ablation catheter (PVAC, Medtronic, Minneapolis, MN), mild PV narrowing (defined by a reduction in diameter of 10-24%) occurred in 35% of PVs, moderate narrowing (defined by a reduction in diameter of 25-50%) occurred in 30% of PVs and severe narrowing (defined by >50% diameter reduction) occurred in 4% of PVs,16 leading the authors to conclude that the observed rates were “a reason for concern.” Our data would suggest that mild to moderate narrowing of the PV diameter (<50%) are within the physiologic range of negative remodeling which can be expected following AF ablation. In the Clinical Study of the Artic-Front Cryoablation Balloon for the Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) trial, using a definition of PVS of reduction in cross-sectional area >75%, only 10 PVs out of 228 patients in the on-treatment analysis met radiographic criteria for PVS.5 Our data suggest that using cross-sectional area, rather than diameter, and taking into account the expected physiologic range of negative remodeling associated with AF ablation, are both likely to improve the sensitivity and specificity of radiographic criteria for determining PVS.
Lastly, it is important to note that our analysis, along with most prior studies assessing the incidence of PVS, have focused on radiographic measurements of PV area and diameter. It is conceivable that beyond area and diameter, other radiographic patterns of PV stenosis may exist, such as unexpected deformities in the shape or morphology of PVs as a result of pathologic injury. Such morphology criteria may be missed when relying on diameter or area alone. However, until such morphologic criteria have been systematically assessed and validated for the purpose of diagnosing PVS, our data suggest that some caution should be applied when using PV diameter and area to identify PVS given the expected ranges of physiologic remodeling.
Several important limitations of our cohort should be noted. First, due to changes over time in the CMR protocol for quantifying LA volumes at our institution, we are unable to assess the relationship between changes in LA volume and PV remodeling. Additionally, although it is interesting to speculate on the relationship between success of ablation (i.e. maintenance of sinus rhythm) and the pattern of PV remodeling, we are unable to provide consistent data on the recurrence rates of AF in this cohort. At our institution, as in many others, there has been a gradual tendency to more rigorous monitoring of patients to detect sub-clinical AF episodes after ablation.3 Therefore, temporal changes in the intensity of monitoring post-ablation would likely confound the relationship between recurrence of AF and PV remodeling patterns. As noted in the Results section, we did not note any correlation between the need for cardioversion post-ablation and the pattern of remodeling.
All follow-up CMRs in this cohort were performed within the first few months after ablation, roughly corresponding to the clinical “blanking period”. Therefore, we are unable to comment on longer-term impacts of ablation on PV remodeling and the extent to which the remodeling patterns observed soon after ablation may change with time. Lastly, our analysis demonstrates a range of physiologic negative remodeling which may impact the diagnosis of PVS when using PV diameter and area criteria. In the absence of histologic or functional assessments of PV remodeling and without specific radiographic criteria to differentiate pathologic injury from physiologic remodeling, we cannot exclude the possibility of overlap between physiologic negative remodeling and pathologic PV stenosis in our cohort. However, as detailed in the Results section, there were no cases of clinically suspected PVS in this analysis.
Pulmonary vein negative remodeling, defined by a reduction in cross-sectional area, occurs in approximately two-thirds of PVs following a first AF ablation and the extent of remodeling follows a roughly bell-shaped distribution with a mean change in PV orifice area of around negative 5-10%. These changes occur in the absence of any clinical evidence to suggest PVS. The expected physiologic range of PV remodeling should be taken into account when determining radiographic thresholds for diagnosing PV stenosis.