Impact of Radiofrequency Ablation of Atrial Fibrillation on Pulmonary Vein Cross Sectional Area: Implications for the Diagnosis of Pulmonary Vein Stenosis
Mohammad-Ali Jazayeria, Subba Reddy Vangaa, Venkat Vuddandaa, Mohit Turagamb, Valay Parikha, Madhav Lavua, Sudharani Bommanaa, Donita Atkinsa, Jayant Natha, Thomas Rosamonda, James Vaceka, Y Madhu Reddya, Dhanunjaya Lakkireddya
aDivision of Cardiovascular Diseases, Cardiovascular Research Institute, Mid America Cardiology, University of Kansas Hospital & Medical Center, Kansas City, KS, USA.bDivision of Cardiovascular Disease, University of Missouri Hospital & Clinics, Columbia, MO, USA.
Restoration of normal sinus rhythm by radiofrequency ablation (RFA) in atrial fibrillation (AF) patients can result in a reduction of left atrial (LA) volume and pulmonary vein (PV) dimensions. It is not clear if this PV size reduction represents a secondary effect of overall LA volume reduction or true PV stenosis. We assessed the relationship between LA volume reduction and PV orifice area pre- and post-RFA.
A retrospective cohort study was conducted at a tertiary care academic hospital. Pre- and post-RFA cardiac computed tomography (CT) studies of 100 consecutive AF patients were reviewed. Studies identifying obvious segmental PV narrowing were excluded. Left atrial volumes and PV orifice cross-sectional areas (PVOCA) were measured using proprietary software from the CT scanner vendor (GE Healthcare, Waukesha, WI).
The cohort had a mean age of 60 ± 8 years, 73% were male, and 90% were Caucasian. Non-paroxysmal AF was present in 76% of patients with a mean duration from diagnosis to RFA of 55 ± 54 months. Mean procedural time was 244 ± 70 min. AF recurred in 27% at 3 month follow-up. Pre-RFA LA volumes were 132 ± 60 ml and mean PVOCA was 2.89 ± 2.32 cm2. In patients with successful ablation, mean LA volume decreased by 10% and PVOCA decreased by 21%. PVOCA was significantly reduced in patients with successful RFA compared to those who had recurrence (2.18 ± 1.12 vs. 2.8 ± 1.9 cm2, p = 0.04) but reduction in LA volume between groups was not significant (118 ± 42 vs. 133 ± 54 ml, p=0.15).
The study demonstrates that both PV orifice dimensions and LA volume are reduced after successful AF ablation. These data warrant a reassessment of criteria for diagnosing PV stenosis based on changes in PV caliber alone, ideally incorporating LA volume changes.
Key Words : Atrial fibrillation, radiofrequency ablation, pulmonary vein stenosis, left atrial volume.
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, affecting 1-1.5% of the population in the developed world, and is associated with a higher incidence of stroke, coronary heart disease, peripheral artery disease, cognitive impairment, and physical disability [1]. Catheter-based radiofrequency ablation (RFA) of AF by pulmonary vein antral isolation (PVAI) is a standard therapy in selected AF patients with symptoms refractory to drug therapy. Restoration of sinus rhythm by RFA has been shown to reduce left atrial (LA) size while preserving function through reverse remodeling, as measured by two-dimensional (2-D) echocardiography [2]-[4], three-dimensional (3-D) echocardiography [5], [6], cardiac computed tomography (CT), and magnetic resonance imaging (MRI) [7], [8].
Pulmonary vein (PV) stenosis is a known complication of PVAI for AF with a variable incidence ranging from 1.3% - 42.4%, depending on technique and operator experience [9], [10]. Increased operator experience and refinement of the RFA technique by moving lesion sites away from an ostial position to focus more on electrical isolation of the surrounding PV antrum has resulted in a dramatic reduction in the incidence of PV stenosis [11]-[13]. The diagnosis of PV stenosis is made based on percentage reduction in PV caliber by a variety of modalities, such as cardiac CT, which can ascertain the PV orifice cross sectional area (PVOCA) for comparison with pre-procedural measurements [12], [14]-[17]. PV stenosis is usually categorized into mild, moderate, or severe stenosis, corresponding to a < 50%, 50 – 70 %, or > 70% reduction in the PVOCA post-ablation, respectively [15].
Even though reduction in LA dimensions post-ablation has been reported in AF patients [3]-[5], [7], there is a relative paucity of data concerning changes in PV dimensions after AF ablation [7]. A decrease in LA volume and improved overall cardiac function can result in decreased pulmonary vascular congestion thereby decreasing the overall PV size [4], [18]. In the absence of a discrete area of stenosis, we attempted to evaluate what percentage of PVs experience a reduction in PVOCA on multi-slice cardiac CT and their relationship to the overall LA volume reduction following restoration of sinus rhythm.
The first 100 eligible AF patients undergoing pulmonary vein antral isolation were enrolled in a retrospective cohort study at a high volume, tertiary care academic medical center.
All patients who had a 64-slice CT scan before and 3-9 months after their first radiofrequency ablation for AF.
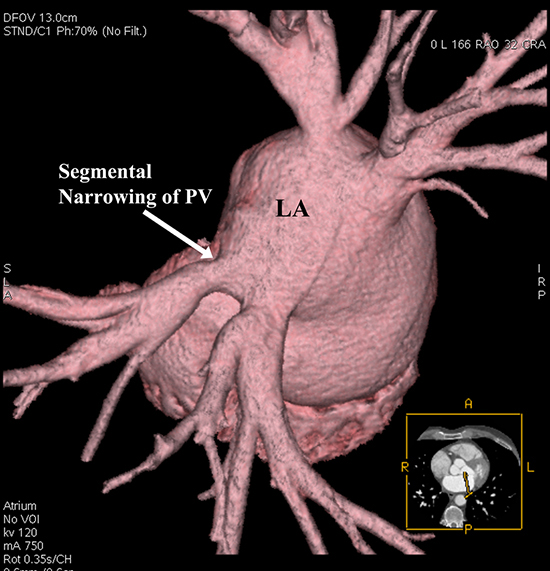
All patients with a history of prior radiofrequency ablation and/or Cox-Maze procedure for AF, and those with a known history of constrictive or restrictive pericarditis were excluded. Patients whose cardiac CT studies showed segmental narrowing of pulmonary veins pre-ablation were also excluded from the study [Figure 1]. Common and middle pulmonary veins were excluded from the analysis. Patients who had MRI studies to evaluate the LA/PV anatomy were also excluded to maintain uniformity in imaging modality. Lastly, patients who developed focal stenosis of the one or more PVs post-ablation were also excluded to purely assess the impact of sinus rhythm restoration on LA and PV dimensions.
The study protocol was approved by the Human Subjects Committee at the University of Kansas Hospital & Medical Center (Kansas City, KS, USA). Baseline, procedural, and outcome data were obtained from our locally maintained institutional atrial fibrillation ablation registry.
Figure 1. Angiography of left atrium from long sheath, positioned in left superior (anatomical right) pulmonary vein
Cardiac CT Studies & Measurements
Patients had cardiac CT images acquired using standard procedure (LightSpeed VCT 64-slice Scanner, GE Healthcare, Waukesha, WI). Heart rate was slowed to about 60 bpm using either oral or intravenous beta-blockers at the time of image acquisition. All patients received iodixanol (Visipaque®, GE Healthcare, Waukesha, WI) using a three-phase protocol. The images were acquired with ECG gating. In patients with AF, images were acquired at 70% of the R-R interval by retrospective gating. In patients who were in sinus rhythm images were acquired before the P wave (at 75% of R-R duration) by prospective ECG gating technology. All cardiac CT scans were manually reviewed by two cardiologists who were blinded to the ablation outcomes.
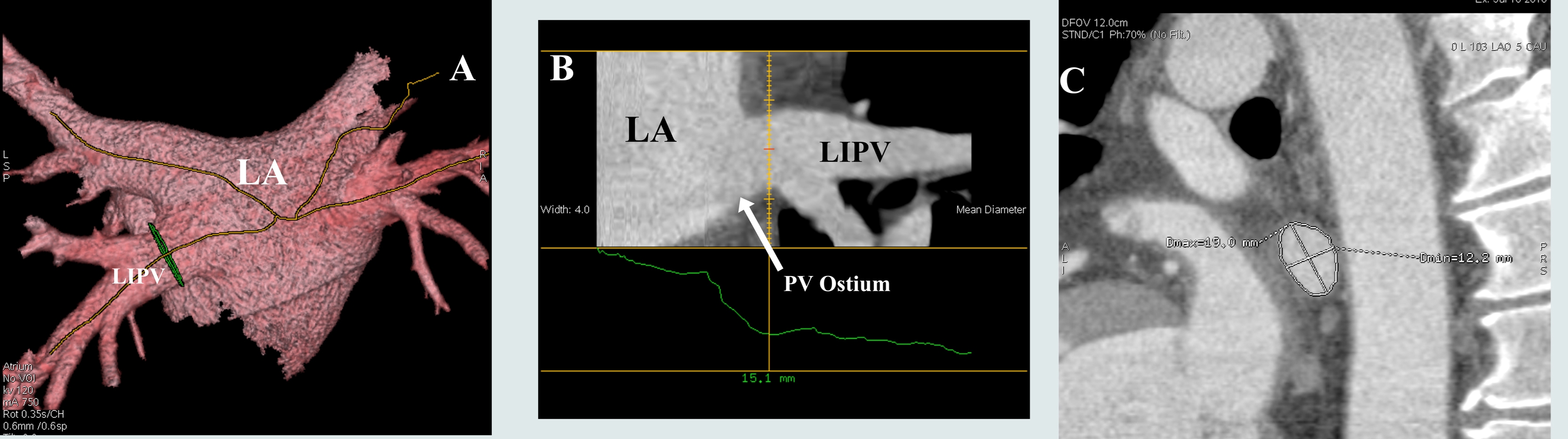
PVOCA was determined at 0.5 cm from each orifice, defined as the point of departure of the vein tangent to the curvature of the LA [17], to provide a uniform pulmonary vein cross-sectional area and to avoid error from any funnel shaped ostia [Figure 2]. Pulmonary vein bifurcation areas were carefully avoided. Using Volume Viewer (GE Advanced Visualization platform) software, LA volume was measured by 3-D reconstruction after defining the region-of-interest by identifying the mitral annulus and pulmonary vein ostia. The left atrial appendage was included in the volume measurements [Figure 2].
Figure 2. Method demonstrating the measurement of the pulmonary vein orifice cross-sectional area. A. Left atrium and pulmonary veins are reconstructed and the axes of the pulmonary veins are marked as lines. B. In this view, the point where the cross-sectional area is to be measured is determined. Care was taken to avoid the funnel shaped ostia and venous bifurcations. C. Once the measurement point has been determined, the cross-sectional area is calculated after marking the entire pulmonary vein cross-section. The picture shows the marked circumference with both major and minor axes. LA: Left atrium; LIPV: Left inferior pulmonary vein; PV: Pulmonary vein.
Atrial Fibrillation Radiofrequency Ablation Procedure
Pulmonary vein antral isolation has been described in detail elsewhere [11], [13], [19]. Briefly, intracardiac echocardiography (ICE) was used to obtain transseptal access and define the anatomy of the pulmonary veins. A circular mapping catheter (Lasso®, Biosense Webster, Inc. Diamond Bar, CA) was used to map around the pulmonary veins and a 3.5-mm irrigated tip catheter NAVISTAR® THERMOCOOL®, Biosense Webster, Inc. Diamond Bar, CA) was used to ablate the PV antra thus achieving electrical isolation. A 3-D map of the LA was reconstructed with CARTO® 3 (Biosense Webster, Inc. Diamond Bar, CA) or Ensite™ NavX™ (St Jude Medical, St. Paul, MN). The procedural end point for this ablation strategy was elimination of all local pulmonary vein potentials along the antra or inside the veins (entrance and exit block). The procedure was performed in patients regardless of presenting rhythm, and, if necessary, direct current cardioversion was performed at the end of the procedure. All patients had therapeutic INRs at the time of the procedure, and additional unfractionated heparin was used to keep the activated clotting time > 400 seconds during the procedure.
All data variables are presented as absolute and relative frequencies. Quantitative variables are expressed as mean ± standard deviation. Paired t-test and Wilcoxon signed-rank test were used to compare paired groups and one-way ANOVA was used to compare 3 groups. All reported p-values are based on two-tailed tests and compared to a significance level of 0.05. All data were analyzed with PASW Statistics for Windows (Version 18.0. Chicago: SPSS Inc.).
The baseline characteristics are shown in [Table 1]. Patients were predominantly Caucasian (92%) with a mean age of 60 ± 8 years and an average body mass index (BMI) of 27 ± 7 kg/m2. Men accounted for 73% (n=73) of the cohort. Many had hypertension (70%) and dyslipidemia (68%). Sleep apnea was observed in 33%, diabetes in 25% and corrected thyroid dysfunction in 19%. A majority of the patients had persistent AF (76%), while permanent and paroxysmal AF were noted in 9% and 24%, respectively. The average AF duration was 55 ± 54 months. The baseline 2-D transthoracic echocardiogram showed an average left ventricular ejection fraction of 57 ± 8% with an LA dimension of 4.3 ± 0.6 cm. On average, the ablation procedure took 244 ± 70 min.
Table 1. Baseline Characteristics
| AF Patients Undergoing PVAI (n = 100) |
| Age (years) |
60 ± 8 |
| Gender (male) |
73 (73%) |
| Race |
Caucasian 92 (92%)
Other 8 (8%) |
| Body Mass Index (kg/m2) |
27 ± 7 |
| Past Medical History
Hypertension
Diabetes
Coronary Artery Disease
Cardiomyopathy
Thyroid Dysfunction
Sick Sinus Syndrome
COPD
Dyslipidemia
Sleep Apnea |
70 (70%)
25 (25%)
21 (21%)
11 (11%)
19 (19%)
11 (11%)
12 (12%)
68 (68%)
33 (33%) |
| Atrial Fibrillation
Type
Persistent
Paroxysmal
Permanent
Mean Duration (months) |
67 (67%)
24 (24%)
9 (9%)
55 ± 54 |
| Pre-ablation 2-D TTE
Ejection Fraction (%)
Left Atrial Size (cm) |
57 ± 8
4.3 ± 0.6 |
| Atrial Fibrillation Ablation
Mean Procedural Time (min)
Fluoroscopy time (min) |
244 ± 70
95 ± 40 |
| Outcomes
Recurrence at 3 months |
27 (27%) |
AF: Atrial fibrillation; PVAI: Pulmonary vein antral isolation; TTE: Transthoracic echocardiogram. All values are expressed as number (percent) or mean ± standard deviation.
Baseline LA volume and PVOCA measurements are shown in [Table 2]. LA volume at baseline differed significantly between paroxysmal and permanent AF (p < 0.0001). The average PVOCA for paroxysmal, persistent, and permanent AF was 2.5, 2.9, and 3.3 cm2 (p=0.41), respectively. Differences in PVOCA among the three types of AF did not reach statistical significance (p = 0.31, 0.31, 0.87) in any of the PVs except in RSPV (p = 0.04).
Table 2. Baseline Cardiac CT Measurements
|
Atrial Fibrillation Type |
|
|
Paroxysmal (n = 24) |
Persistent (n = 67) |
Permanent (n = 9) |
p-value |
| LA Volume (ml) |
110.2 ± 29.5 |
128.5 ± 32.0 |
214.0 ± 159.5 |
<0.0001 |
| RIPV (cm2) |
2.6 ± 1.2 |
3.4 ± 2.5 |
3.5 ± 2.5 |
0.3104 |
| LIPV (cm2) |
2.0 ± 1.1 |
2.3 ± 0.8 |
2.4 ± 0.8 |
0.3070 |
| RSPV (cm2) |
3.0 ± 1.2 |
3.4 ± 2.1 |
4.9 ± 2.1 |
0.0439 |
| LSPV (cm2) |
2.4 ± 1.1 |
2.6 ± 1.8 |
2.5 ± 1.1 |
0.8696 |
| Average of all PVs |
2.5 ± 1.1 |
2.9 ± 1.8 |
3.3 ± 1.6 |
0.4058 |
LA: Left atrium; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein. All values are expressed as mean ± standard deviation.
Effect of RF ablation on LA volume & PVOCA
[Table 3] summarizes the baseline and post-procedural cardiac CT measurement data. Successful RF ablation resulted in a dramatic reduction in post-ablation PVOCA compared to the pre-ablation values (2.89 ± 2.32 cm2 vs. 2.18 ± 1.12 cm2, p = 0.04). Patients who remained in AF after PVI (n = 27) did not demonstrate a significant change in LA volume or PV ostial cross-sectional area from baseline. When sinus rhythm was restored, the LA volume decreased by 11% (p = 0.15). On average, the PV cross-sectional areas was decreased by 22%.
Table 3. Post-procedural Cardiac CT Measurements Grouped by Ablation Outcomes
| ± |
Pre-ablation (n = 100) |
Post-ablation AF Recurrence (n = 27) |
Post-ablation Successful (n = 73) |
Percentage Change with Restoration of Sinus Rhythm |
p-value |
| LA Volume (ml) |
132 ± 60 |
133 ± 54 |
118 ± 42 |
11% |
0.15 |
| Pulmonary Vein Cross Sectional Areas (cm2) |
| Left Superior PV |
2.5 ± 1.6 |
2.4 ± 1.5 |
1.8± ± 1.0 |
25% |
0.02 |
| Left Inferior PV |
2.2 ± 0.9 |
2.3 ± 0.9 |
1.8 ± 0.8 |
21% |
0.01 |
| Right Inferior PV |
3.2 ± 2.3 |
3.0 ± 1.3 |
2.5 ± 1.2 |
17% |
0.07 |
| Right Superior PV |
3.4 ± 2.0 |
3.4 ± 1.5 |
2.6± ± 1.2 |
18% |
0.01 |
| Average |
2.89 ± 2.32 |
2.80 ± 1.9 |
2.18 ± 1.12 |
22% |
0.04 |
LA: Left atrium; PV: Pulmonary vein. All values expressed as mean ± standard deviation
Data related to subgroup analyses are displayed in [Table 4]-[Table 6]. In the subgroup of patients in whom sinus rhythm was successfully restored, post-ablation follow-up CT scan measurements generally showed a trend towards smaller volumes and cross-sectional areas [Table 4]. In paroxysmal AF patients who successfully maintained sinus rhythm, LA volumes were reduced by 11% (p = 0.30). In the persistent AF group, LA volumes were reduced by 13% (p = 0.02), and in permanent AF group, the reduction was 9% (p = 0.58). The average PV cross-sectional areas were reduced by 16% (p = 0.26), 30% (p = 0.004), and 23% (p = 0.38) in the paroxysmal, persistent, and permanent AF groups, respectively [Table 5].
In the subgroup of patients where AF persisted despite ablation, the change in LA volumes and PV cross-sectional areas were relatively unaffected. The mean LA volume was reduced by 3% in paroxysmal AF (p = 0.86), 2% in persistent AF (p = 0.76) and 9% in permanent AF (p = 0.85). The individual PV cross-sectional areas remained relatively unchanged as well [Table 6].
Table 4. Percentage Reduction of All Individual Pulmonary Veins
|
LA Volume (n = 100) |
LSPV (n = 97) |
LIPV (n = 96) |
RIPV (n = 98) |
RSPV (n = 99) |
All PVs (n = 390) |
| >25% reduction |
13 (13%) |
52 (54%) |
31 (32%) |
30 (31%) |
31 (31%) |
144 (37%) |
| 0-25% reduction |
65 (65%) |
25 (26%) |
35 (36%) |
43 (44%) |
37 (37%) |
140 (36%) |
| No reduction or increase in size |
22 (22%) |
20 (21%) |
30 (31%) |
25 (26%) |
31 (31%) |
106 (27%) |
LA: Left atrium, LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein; PV: Pulmonary vein. All values are expressed as number (percentage). The percentage reduction of each vein was calculated compared to preablation CT. They were grouped into 3 groups depending the amount of change noticed (>25%, 0-25% reduction, no reduction or increase). A total of 390 veins were analyzed, common or middle pulmonary were not included in the analysis.
Table 5. Subgroup Analysis of Patients with Successful Restoration of Sinus Rhythm
| Sinus Rhythm Restored Group (n = 73) |
| Baseline AF Type |
Paroxysmal (n = 15) |
Persistent (n = 52) |
Permanent (n = 6) |
| ± |
Pre-ablation |
Post-ablation |
% Change (p-value) |
Pre-ablation |
Post-ablation |
% Change (p-value) |
Pre-ablation |
Post-ablation |
% Change (p-value) |
| LA Volume (ml) |
114 ± 25 |
102 ± 36 |
11% (0.30) |
132 ± 37 |
115 ± 38 |
13% (0.02) |
151 ± 47 |
137 ± 38 |
9% (0.58) |
| LSPV (cm2) |
2.50 ± 1.26 |
2.39 ± 0.78 |
4% (0.78) |
2.50 ± 1.69 |
1.72 ± 0.88 |
31% (0.004) |
2.72 ± 1.27 |
2.38 ± 1.69 |
13% (0.70) |
| LIPV (cm2) |
2.24 ± 1.11 |
1.68 ± 0.42 |
25% (0.08) |
2.25 ± 0.83 |
1.64 ± 0.71 |
27% (0.0001) |
2.47 ± 1.00 |
1.82 ± 0.65 |
55% (0.21) |
| RIPV (cm2) |
2.65 ± 1.04 |
2.38 ± 0.96 |
10% (0.47) |
3.46 ± 2.79 |
2.32 ± 1.30 |
34% (0.009) |
3.84 ± 3.06 |
3.07 ± 2.03 |
20% (0.62) |
| RSPV (cm2) |
2.82 ± 0.85 |
2.10 ± 1.23 |
26% (0.07) |
3.32 ± 2.31 |
2.38 ± 1.04 |
28% (0.009) |
5.53 ± 2.08 |
3.87 ± 0.83 |
30% (0.10) |
| Average |
2.55 ± 1.07 |
2.14 ± 0.85 |
16% (0.26) |
2.88 ± 1.91 |
2.01 ± 0.98 |
30% (0.004) |
3.64 ± 1.85 |
2.79 ± 1.3 |
23% (0.38) |
AF: Atrial fibrillation; LA: Left atrium; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein; PV: Pulmonary vein. All values are expressed as mean ± standard deviation.
Table 6. Subgroup Analysis of Patients Remaining in AF after Radiofrequency Ablation
| AF Recurrence Group (n = 27) |
| Baseline AF Type |
Paroxysmal (n = 9) |
Persistent (n = 15) |
Permanent (n = 3) |
| ± |
Pre-ablation |
Post-ablation |
% Change (p-value) |
Pre-ablation |
Post-ablation |
% Change (p-value) |
Pre-ablation |
Post-ablation |
% Change (p-value) |
| LA Volume (ml) |
107 ± 34 |
104 ± 35 |
3% (0.86) |
121 ± 18 |
119 ± 17 |
2% (0.76) |
185 ± 104 |
169 ± 99 |
9% (0.85) |
| LSPV (cm2) |
2.14 ± 0.74 |
1.74 ± 0.73 |
19% (0.27) |
2.74 ± 2.04 |
2.32 ± 1.29 |
15% (0.51) |
2.09 ± 0.70 |
1.90 ± 0.65 |
9% (0.75) |
| LIPV (cm2) |
1.55 ± 1.03 |
1.73 ± 0.92 |
-12% (0.70) |
2.29 ± 0.76 |
2.08 ± 0.99 |
9% (0.52) |
2.17 ± 0.65 |
2.23 ± 0.85 |
-3% (0.03) |
| RIPV (cm2) |
2.57 ± 1.59 |
2.25 ± 0.89 |
12% (0.61) |
3.23 ± 1.09 |
2.86 ± 1.3 |
11% (0.41) |
3.04 ± 1.61 |
3.00 ± 1.17 |
1% (0.97) |
| RSPV (cm2) |
3.17 ± 1.71 |
2.79 ± 0.97 |
12% (0.52) |
3.49 ± 1.31 |
2.9 ± 1.36 |
17% (0.24) |
3.78 ± 1.91 |
3.76 ± 0.64 |
1% (0.99) |
| Average |
2.36 ± 1.27 |
2.13 ±± 0.88 |
10% (0.66) |
2.94 ± 1.3 |
2.54 ± 1.2 |
14% (0.39) |
2.77 ± 1.22 |
2.72 ± 0.83 |
2% (0.96) |
AF: Atrial fibrillation; LA: Left atrium; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein; PV: Pulmonary vein. All values are expressed as mean ± standard deviation.
Our study confirms that, following RF ablation of atrial fibrillation, restoration of sinus rhythm reduces LA volume and PV orifice cross-sectional areas physiologically. We believe the reduction in LA volume and PV dimensions are a direct consequence of RF ablation and sinus rhythm restoration. Care was taken to avoid including patients whose pulmonary veins showed segmental narrowing, which may indicate a pre-existing anomaly or direct injury to the pulmonary vein from RF energy delivery. In addition, conditions that could potentially limit or restrict changes in LA volume were excluded from the study. Therefore we believe the exclusion criteria resulted in a cohort in whom the effect of sinus rhythm restoration could be studied in relative isolation.
The results confirmed that a reduction in LA volume, in the absence of focal stenosis, is associated with a concomitant reduction in PV caliber, as assessed by ostial cross-sectional area. The diagnosis of PV stenosis is typically made based upon comparison with pre-ablation cardiac CT dimensions [12], [14]-[16], [20]. Our study demonstrates that following successful AF ablation, a reduction in cross-sectional area of up to 25% may be observed in some pulmonary veins. We believe that reduction of this magnitude without any evidence of focal stenosis should be considered physiologic and likely a distinct, compensatory process in comparison to the pronounced pathologic changes of PV stenosis. Asymptomatic patients may therefore be spared additional cardiac CT studies, thereby reducing their radiation exposure in addition to healthcare expenditures.
At baseline, LA volumes were significantly different among the different AF type groups. Previous studies have shown similar changes in LA diameter and volume after AF ablation [2]-[4], [6], [7], [21]-[23]. The precise mechanisms resulting in the observed LA volume and PVOCA reductions could not be established in this study. Multiple etiologies have been postulated to explain the volume reduction, including reverse remodeling of the LA, scar tissue resulting from RF thermal injury, improved LA function, and reduced LA pressures [4], [6], [18].
Our results are consistent with other studies which have shown similar reductions in LA size only in patients seemingly free of AF at follow-up [3], [22], suggesting positive remodeling of LA after successful sinus rhythm restoration. Other studies have shown reduction in LA volume irrespective of sinus rhythm restoration [4], [6], [7]. This could be due to fibrosis from scarring of the LA from RF injury rather than positive remodeling. A more nuanced explanation might involve a correlation between AF burden and pre-ablation LA size, as suggested in the findings of Jayam et al. who found that in a multivariate regression only pre-ablation LA volume predicted the percentage reduction in LA volume post-ablation [7]. Indeed, this seems consistent with our finding of significant LA volume differences among paroxysmal, persistent, and permanent AF patients at baseline [Table 2].
Furthermore there appears to be an important relationship between the reduction in LA volume and PVOCA post-ablation and AF chronicity, with a significant benefit seen in early persistent AF patients as compared to those with paroxysmal AF. Interestingly, though the improvement in the long standing persistent or permanent AF patients was higher than that seen in the paroxysmal AF group, it was less than pronounced than the early persistent group. This observation highlights the untoward effects of progressive AF and also suggests the possibility of permanent remodeling changes that could result in irreversible scar formation in this subgroup of patients. Further clinical and laboratory investigation promises to help elucidate these mechanisms further.
Our study has a number of limitations that merit discussion. The time span from restoration of sinus rhythm to completion of the follow-up CT scan is important, as it can affect the observations related to LA remodeling and fibrosis and affect comparisons between various subgroups. LA pressure changes can alter the LA size and shape, and unfortunately there are no reliable, non-invasive methods to accurately assess LA pressure. The amount of LA fibrosis can affect the the chamber’s elasticity and prevent positive or reverse remodeling of the LA after restoration of sinus rhythm. Quantification of post-ablation LA scarring and fibrosis for comparison to pre-ablation findings would have helped us to differentiate the relative contributions of increased LA scarring and/or positive remodeling to the observed changes in LA and PVOCA dimensions. However, due to technical limitations with our institutional MR scanners, we could not assess the LA scar reproducibly, which precluded this endeavor. Lastly, our study is limited by small size, particularly in the paroxysmal and permanent AF subgroups. In these subgroups, although there was a trend of decreased LA volume and PVOCA after restoration of sinus rhythm, it did not reach statistical significance, which may have been circumvented with a larger sample size.
The study demonstrates that both PV orifice cross-sectional area and LA volume are reduced after successful AF ablation. These data warrant a reassessment of criteria for diagnosing PV stenosis based on changes in PV caliber alone, ideally incorporating adjustments to reflect reductions in LA volume post-ablation. Further studies are needed to identify the role of LA pressure, remodeling and fibrosis in predicting LA and PV dimension changes, as they may also enhance our understanding of the pathophysiology of pulmonary vein stenosis and inform techniques to prevent this complication.
None of the authors have any disclosures relevant to the study.