Intra-Atrial Block: Definition and Relationship to Atrial Fibrillation and Other Adverse Outcomes
James A. Reiffel1
1Columbia University
c/o 202 Birkdale Lane
Jupiter, FL 33458 U.S.A
.
In 1916, Bachmann first reported on the inter-auricular time interval. However relatively little attention was paid to this ECG measurement for decades. Then, in 1956, Samuel Bradley and Henry JJ Marriott reported on intra-atrial block (IAB) in 4,500 ECGs.As defined by them, IAB was a P wave duration of 0.12 sec or longer. Since that time, others have defined IAB as 0.11 sec or longer or 0.12 sec or longer. Several authors have suggested subcategories, such as first-, second-, and third-degree patterns and some have defined specific intra-atrial and inter-atrial pathways. These are of electrocardiographic interest but have not been substantiated as related to different clinical outcomes. Many disorders have been associated with IAB. More importantly, however, IAB has been associated with several adverse outcomes, including sinus node dysfunction, atrial tachyarrhythmias – especially atrial fibrillation, thromboembolic events, and increased mortality. This brief review will detail the above to emphasize to ECG readers the importance of not overlooking IAB in their interpretations.
Key Words : Intra-atrial block, Inter-atrial block, atrial conduction, P wave duration Running Head, Intra-Atrial Block: Recognition and Significance.
Correspondence to: James A. Reiffel, M.D.
Columbia University
c/o 202 Birkdale Lane
Jupiter, FL 33458 U.S.A.
Recently, in an ECG case in Circulation, Qin et al.[1]reported a 2:1 pattern of intra-atrial block (IAB) [a topic discussed in the literature much less frequently than other conduction abnormalities] as a cause of alternating P wave morphology. It was a beautiful example of normal sinus P waves alternating with widened and notched sinus P waves. However: (1)I was dismayedby the specific referenceschosen to define IAB and its patterns and incidence, since each reference related only to intra-atrial conduction impairment following a catheter ablation procedure, which was not a factor in their patient’s history or care. (2) I was surprised that they used post-ablation intervals to defineintra-atrial conduction in a non-ablated patient rather than information from the long history of reports regarding the definition and patterns of IAB in the absence of ablation. (3) They oversimplified the P wave variations that can occur with IAB. And, (4) I wondered why they did not also discuss the importance of IAB with respect to adverse outcomes – after all, it is more than just an ECG curiosity. This allsuggested to me that a concise review of IAB would be timely.
In 1916, Bachmann first reported on the inter-auricular time interval. [2] However relatively little attention was paid to this ECG measurement for decades. Then, in 1956, Samuel Bradley and Henry JJ Marriott reported on intra-atrial block in 4,500 ECGs.[3] As defined by them, IAB was a P wave duration of 0.12 sec or longer. Notching, as was seen by Qin et al.[1] occurred in ~10% of IAB. Moreover, Bradley and Marriott critically examined the even earlier literature that considered definitions of >0.10 sec, 0.11 sec, and 0.12 sec and concluded that there was good justification for adopting 0.11 sec as the upper limit of the normal P wave and for calling IAB as a sinus P wave of 0.12 sec or longer. In their 4,500 ECGs, the incidence of IAB was 4.5% -- “almost as high as that of atrioventricular or intraventricular block in the same series.” More recently, Fauchier et al. [4] demonstrated that in patients with sinus node dysfunction, AV conduction disturbances, paroxysmal supraventricular tachycardias, and paroxysmal atrial fibrillation with slow ventricular responses, the incidence of IAB is even higher, being 26%, 20%, 16%, and 31% respectively. Others, including Antonio Bayes de Luna in 2015, agreed with the 0.12 sec definition for IAB, though some, including David Spodick and colleagues in 2014 and Williams and colleagues in 2015 defined IAB as a P wave of 0.11 sec or longer. [5,6,7]
Subsequent to the work of Bradley and Marriott, Jules Cohen and David Scherf detailed the patterns of complete intra-atrial and inter-atrial block,[8] which included intra-atrial and inter-atrial dissociation (separate rhythms within one atrium or between the two atria) and related atrial conduction impairment that protects atrial parasystolic foci. Moreover, Thomas James and others attempted to detail the pathways of preferential conduction within the right atrium from the sinus node to the AV node and between the right atrium and the left atrium [9-16] in which conduction delays would result in IAB and often associated alterations in P wave morphology. Intra-right atrial conduction, according to such investigators, [9-11] occurs primarily via three preferential pathways (anterior, middle, and posterior), although they have not been as convincingly identified histologically as are the intraventricular and His-Purkinje conduction tissues. Right atrial to left atrial conduction appears to occur most often via Bachmann’s bundle, though less frequently (and less well studied and characterized) it may occur via fibers in or around the fossa ovalis or coronary sinus. [12-14] Such left atrial breakthrough sites have been most recently confirmed in patients undergoing ablation for atrial fibrillation using magnetocardiography and electroanatomic mapping. Notably, IAB appears to be more frequent in patients with atrial fibrillation who are evaluated at the time of ablation than it is in patients without atrial fibrillation.Consider, however, that this data may reflect a selection bias as those patients with atrial fibrillation who are referred for ablation tend to be those whose atrial fibrillation has not been medication-responsive and thus may have more advanced altered atrial electrophysiology and/or more advanced atrial histopathology. They tend to have wider P waves than those patients without atrial fibrillation (see below).
To perhaps more precisely characterize IAB, Bayes de Luna and others[5,6,10,15-17] attempted to subdivide IAB into first-, second-, and third-degree patterns, analogous to the approach taken for AV conduction disturbances. In its simplest terms, first degree IAB is a widened P wave, with or without notching; second degree is abrupt and transient P wave widening, perhaps most often with delay in Bachmann’s bundle, and third degree is IAB with loss of conduction across Bachmann’s bundle. [Figure 1] schematically shows normal intra- and inter-atrial conduction, IAB conduction, and IAB with block in Bachmann’s bundle. [Not shown are possible delays in the inter-atrial connections via the coronary sinus or fossa ovalis regions.] Notably, several authors have suggested particular P wave alterations on ECGs or vectorcardiograms during sinus rhythm that are suggestive for specific locations of intra-atrial block [11,15,18-20], with those associated with delayed left atrial breakthrough most likely to be associated with risk for atrial fibrillation. [Readers who are interested in the specific P wave alterations are referred to these references for more detail.] See [Figure 2] for ECG examples of several patterns of IAB.
Figure 1. A schematic of normal intra and inter atrial conduction (solid arrows represent normal conduction velocity), panel A; slow intra and inter atrial conduction – represented by the squiggly lines, panel B; and slow intra atrial conduction with block across Bachmann’s bundle, panel C. Conduction across the less frequently apparent coronary sinus (CS) and fossa ovalis (FO) fibers, which are likely used to achieve conduction to the left atrium in the setting of Bachmann’s bundle block, is not illustrated although their approximate locations with respect to Bachmann’s bundle is shown.

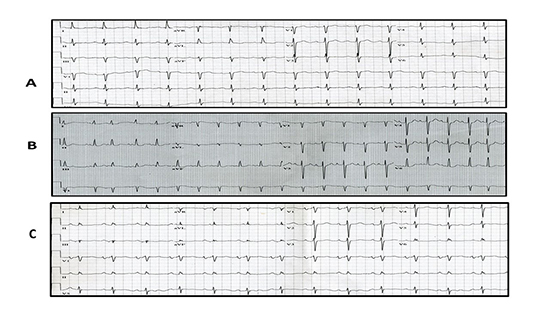
Figure 2. Three examples of IAB. Panel A shows a widened but smooth P wave. Panel B shows a widened and notched P wave (as well as some premature atrial complexes). Panel C shows a widened and notched P wave with the terminal forces being negative in the inferior leads. Panel A is most compatible with delay mainly in the right atrium. Panel B is most compatible with delay between the right and left atria, with the second part of the P wave (second notch) most likely representing delayed left atrial activation. Panel C is most suggestive of delay in Bachmann’s bundle, with the terminal forces going away from the inferior leads and upwards towards the left atrium due to lower septal activation rather than conduction across Bachmann’s bundle in the upper septum.
While IAB patterns are interesting electrocardiographically, subdividing IAB does not appear exceptionally helpful to me clinically, other than recognizing an increased association with atrial fibrillation when left atrial breakthrough is affected. Notably, the association of IAB (particularly when it is advanced as a consequence of delay in Bachmann’s bundle as manifest by negative terminal forces in the inferior leads suggestive of retrograde left atrial activation) and atrial fibrillation has been termed Bayes syndrome. [11,15] Personally, I would think that inter-atrial block with independent right and left atrial rhythms or intra-atrial block with two separate atrial rhythms within the same chamber would be a better definition for third degree IAB [Figure 3]. Perhaps, first degree IAB could be subcategorized by the presumed pathway(s) with delay or block. Importantly, with respect to Bayes syndrome, the block in Bachmann’s bundle not only results in delay of left atrial activation but altered routes to left atrial activation, which could result in the potential for additional reentrant pathways within the atria (perhaps especially if Bachmann’s bundle block is only unidirectional) thereby facilitating the development of atrial tachyarrhythmias.
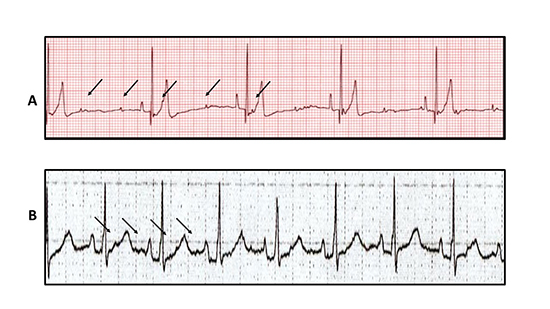
Figure 3. Two tracings showing types of atrial dissociation. Panel A shows right and left atrial dissociation with independent rhythms and therefore two sets of P waves marching through each other. Only the right atrial P wave (sinus rhythm) conducts to the ventricles and results in QRS complexes. Panel B shows a similar phenomenon but in this case the patient is post heart transplant with one set of P waves conducting to the ventricles that originate in the transplanted right atrium and the second set comingfrom the recipient’s atrial rim. Arrows are place to indicate the non-conducted P waves.
Regardless of the site and degree of IAB (other than inter-atrial dissociation), but of importance because IAB widens the P wave and the measurement of the PR interval begins with the onset of the P wave, IABcan result in a lengthened PR interval through delay in impulse conduction from the sinus node, through the right atrium, to the AV node. In this circumstance, PR prolongation does not indicate any AV conduction delay, as it results from widening of the P wave and lengthening of the PA interval (as seen on intracardiac tracings) rather than prolonging the AH interval which is indicative of AV nodal delay. In contrast, if IAB is a consequence of right atrial to left atrial delay, but intra-right atrial conduction velocity is normal, then PR prolongation should not be noted. Importantly, IAB and sub-atrial conduction disturbances can coexist. Notably, Qin et al.[1] essentially ignored the contribution of the IAB, when present, to the prolonging of the associated PR interval in their report. In their case of 2:1 IAB, with the PR interval being normal when the P wave width was normal, the IAB-associated prolonged PR interval clearly did not indicate delay of AV conduction.
In addition to defining IAB Bradley and Marriott [3] also explored the causes of P wave prolongation, as have others.[7,11] Reported causesinclude disorders that result in left atrial enlargement [including mitral stenosis and the P-mitrale pattern and many left ventricular disorders];Chagas disease; inflammatory and infiltrative disorders; age-related fibrosis; atrial septal structural abnormalities; and atrial ischemia (via impaired flow through Condorelli’s artery – the left anterior atrial artery which supplies Bachmann’s bundle among other atrial areas). Other causes include medications, such as quinidine and digitalis, as well as vagal stimulation. Recently, it has been shown that IAB may be provoked by adenosine; reduced by atropine;can be functional, occurring or stopping after refractory period changes associated with premature impulses; and may be associated with obstructive sleep apnea. [6,11,2-10,16,17,21,22,24] Sometimes the P wave morphology can offer a clue as to possible contributors to IAB or the site of delay. For example, a left atrial enlargement pattern would suggest left heart pathology. Negative terminal P wave forces in the inferior leads with a P wave otherwise compatible with sinus rhythm can suggest impairment of atrial impulse transmission across the upper atrial septum (e.g., Bachmann’s bundle) resulting in lower transseptal conduction with resulting inferior to superior terminal P wave forces ([Figure 2], panel C). Because the interested reader can find substantial information regarding the electrophysiological, anatomic, and ultra-structural alterations that can underly IAB in other prior publications [6,7,15] I will not detail them in this brief review.However, it is of note that they do include cell loss, intercellular collagen deposition with the widest P waves being associated with the greatest amount of collagen deposition, [6,17] as well as excessive stretch of atrial myocardium. Of note, such changes can affect intercellular conduction and serve as a basis for reentrant circuits as well as impairment of function of atrial-associated tissues, such as the sinus node. Also, of note, IAB may be the only manifestation of an atrial disorder in some patients whereas in others when disease affects not only intra-atrial conduction but also myocyte function and therefore atrial contractility and possibly size, IAB is then part of an atrial cardiomyopathy. In some of the latter cases, progressive disease can ultimately result in atrial standstill with slow junctional escape rhythms. More frequently in others, the underlying atrial pathology as well as the associated atrial conduction impairments result in altered atrial electrophysiology and atrial tachyarrhythmias, such as atrial fibrillation. When the underlying disorders are associated with risk markers for thromboembolism in the presence of atrial fibrillation, such as hypertension, diabetes, heart failure, advanced age, then prophylactic oral anticoagulation is appropriate to consider. Thus, IAB should indicate the need for both an evaluation of any associated disorders and a closer follow up regarding atrial fibrillation (which in many patients has been shown to be subclinical but detectable by appropriate monitoring, prior to its first clinical presentation).
Lastly, in the era of transvenous catheter-based human electrophysiologic studies, confirmation of the above noted ECG-based interpretations has occurred. In 1977, our group demonstrated that IAB can be rate-related, as was shown in a case of simultaneous intra-atrial and intra-ventricular conduction defects that mimicked an intermittent trifascicular conduction disorder. [25] Others have shown that: (1) portions of the right atrium can be in sinus rhythm (protected by entrance block) while other portions and the left atrium can be in flutter-fibrillation; (2) similar phenomena can exist in the left atrium following ablation; (3) intra-atrial block may occur during atrial tachycardia thoughbeing absent during sinus rhythm; (4) intra- and inter-atrial dissociation can occur during atrial flutter or fibrillation,[26-33] and (5) second degree IAB can be produced with atrial stimulation at critical drive rates (in an era well before ablation) thus confirming rate-related potential.[34] In one patient, electrophysiologic testing of sinus node function in the small section of the right atrium that was in sinus rhythm revealed an underlying sinus node dysfunction, while the rest of the right atrium and the left atrium was in atrial flutter-fibrillation. [26]
Why should we care about IAB? After all, it is just widening of the P wave. Isn’t this just cosmetic?To the contrary.
First, as noted earlier, IAB can mimic delayed AV conduction. Since the P wave onset initiates the measurement of the PR interval, IAB can result in PR prolongation, which may then be misinterpreted as delayed AV nodal and/or His-Purkinje conduction. Second,in patients with IAB, likely as a marker of atrial disease but also as an indicator of areas of delayed conduction in the atria that might sub serve reentry, there is an increased likelihood for development of atrial tachyarrhythmias, most frequently atrial fibrillation (AF) as well as for recurrences of AF following both cardioversion and ablation.This has been noted in multiple reports [5,6,11,17,35-41] and may be the most important associated consequence of IAB (as well as the reason IAB should be of interest to readers of this journal). Less well studied is the relationship between IAB and the risk of post-ablation atrial tachycardias. Of note, but perhaps not of surprise, the risk for developing AF appears greatest when IAB is most pronounced, as measured by P wave width or P wave dispersion. [11,13-14,20,26-32,35-46]Relatedly, there have been reports of an increased risk for thromboembolism in patients with IAB—both stroke and peripheral. [6] Third, in our experience, not infrequently IAB also accompanies sinus node dysfunction, thus likely indicating pathology of both the sinus node and surrounding atrial tissue.Interestingly, in at least one report, in patients with sick sinus syndrome and paroxysmal atrial fibrillation in whom right atrial pacing was instituted as part of preventive treatment for recurrent AF, the presence of IAB during sinus rhythm was associated with a higher incidence of recurrent AF than when IAB was absent. [47] Accordingly, IAB may caution the clinician to be alert for symptoms compatible with sinus node dysfunction as well as atrial tachyarrhythmias; and, due to the latter, may signal a need for closer follow up by the treating physician. Moreover, IAB may be appropriate to consider in association with a patient’s demographic and echocardiographic profile with respect to selection of patients to monitor for subclinical AF.Fourth, IAB has been epidemiologically associated with increased cardiovascular and total mortality. [6,48]Fifth, though rarely, severe IAB can result in cessation of atrial tachycardias that were previously present but dependent upon lesser but critical degrees of intra-atrial conduction delays. [49] Sixth, inter-atrial block can result in atrial dissociation with independent atrial rhythms that may be symptomatic and may confuse the interpretation of the standard ECG. [26,50-52]
With specific respect to intermittent IAB or varying degrees of IAB rather than consistent P wave widening, in 1974 MA Legato and MI Ferrer reportedon the diagnosis, incidence, and implications of intermittent IAB.[53] These authors noted that with intermittent IAB: (1) the PR interval was significantly shorter when the P wave duration was normal but that the PR interval lengthened by a smaller increment than that which widened the P wave itself, suggesting that some of the P wave delay can occur after the onset of AV nodal activation; (2) intermittent IAB was not typically related to a change in sinus rate; and, (3) in 16 of their 56 study patients, intermittent IAB was the only defect on the ECG (18%) except for rare ectopy. In the other 40, while there were additional ECG abnormalities present, there was no consistent ECG finding that associated with the IAB. They also reported that the most attractive explanation for intermittent IAB are abnormalities in the specialized internodal tracts (e.g., per James et al) and/or inter-atrial tracts (e.g., Bachmann’s bundle), with the latter being accompanied by alterations in the P wave morphology (such as a negative terminal component to the sinus P wave in the inferior leads). They supported their observations with literature involving lesions placed within specific atrial regions. In subjects with the most marked P wave widening (more than 30 msec), they invoked atrial myofibrillar blocks – in the working myocytes) beyond simple delay in the inter-nodal or inter-atrial tracts. However, as noted earlier, the most marked P wave widening may be related to more marked intercellular collagen deposition and not just myocyte alterations. Finally, in following their subjects, Legato and Ferrer reported that many of their patients developed non-intermittent IAB over several months to years as well as a variety of atrial arrhythmias, including permanent atrial fibrillation or slow non-sinus atrial rhythms “at an incidence greater than would be expected for a population of comparable age and background”.[53] These follow up observations are compatible with those noted by others above and again suggest that IAB may be a harbinger of later AF.
Table 1. Study and patient characteristics
| Study |
Country |
Age (yr)* |
Sex |
AF type |
LAAO type |
LAAO size (mm) |
CHA2DS2VASC |
Reason for LAAO |
Implantation |
Diagnosed |
Presenting Symptoms/Signs |
LAA characteristics |
Injury |
LAA and vessel relation |
Management |
Outcome |
| Pulmonary artery Injury |
| Scislo et al 2018 (1st case) |
Poland |
67 |
F |
Parox |
Amulet |
25 |
9 |
GI and intracranial bleeding |
No issue |
17 hrs post procedure |
Chest pain, dyspnea, hemodynamic collapse |
Winsock type, LZ-20mm |
3mm posterolateral tear to MPA by anchoring hook |
2mm groove between MPA and LAA |
Thoracotomy and repair |
Discharged alive |
| Scislo et al 2018
(2nd case)
|
Poland |
62 |
M |
Parox |
Amulet |
28 |
3 |
GI bleeding |
No issue |
3hrs post-procedure |
Cardiac tamponade |
Winsock type,
LZ 23mm
|
3mm posterolateral tear of MPA by anchoring hook |
No groove between MPA and LAA |
Thoracotomy and repair |
Discharged alive |
| Wang et al 2018 |
Australia |
87 |
M |
Persis |
Amulet |
31 |
6 |
Hemorrhagic stroke |
No issue |
6months post-procedure |
Cardiac tamponade and collapse |
LZ 25mm |
2mm posterolateral tear from chronic pressure ofanchoring hook without erosion of LAA |
- |
Thoracotomy and repair |
Discharged alive |
| Suwalski et al 2016 |
Poland |
66 |
M |
Persis |
ACP |
22 |
- |
Intracranial hemorrhage |
No issue |
17 days post-procedure |
Cardiac Tamponade |
LAA origin 18mm, depth 24mm |
2mm lateral surface tear by anchoring hook |
- |
Thoracotomy and repair |
Discharged alive |
| Bianchi et al 2013 |
Italy |
76 |
M |
Persis |
ACP |
22 |
3 |
Intracranial hemorrhage |
No Issue |
3hrs post procedure |
Cardiac tamponade and collapse |
- |
2mm tear by anchoring hook |
- |
Thoracotomy and repair |
Discharged alive |
| Sepahpour et al 2013 |
Australia |
72 |
F |
Parox |
Watchman |
24 |
6 |
Complicated PCI and need for lifelong dual antiplatelet |
Transient inf STEMI, no apparent reason and resolved by reimplantation with 2nddevice |
16 days post procedure |
Shock, PEA, could not be resuscitated |
LAA orifice diameter 17mm, depth 35mm |
10mm tear on superior and left aspect of MPA by a metallic strut of Watchman |
- |
Died |
Died |
| Zwirner et al 2016 |
Germany |
71 |
F |
Persis |
Amulet |
- |
- |
Traumatic subdural hemorrhage |
No issue |
8hrs post-procedure |
Patient was found pulseless, failed resuscitation |
- |
2mm tear on the pulmonary artery by a hook of amulet with punctiform tear of LAA |
- |
Died |
Died |
| Hanazawa et al 2014 |
Germany |
75 |
F |
Parox |
ACP |
24 |
5 |
Subdural hematoma |
No issue |
24hrs post-procedure |
Hypotensive, cardiac tamponade |
LAA orifice diameter 18mm, depth 27mm |
Perforation of LAA and leading to erosion of the bottom of the pulmonary artery |
3D reconstruction of cardiac CT showed one lobe of LAA touched the inferior pulmonary artery |
Died |
Died |
| Pulmonary Vein Compression |
| Ayati et al 2014 |
Germany |
76 |
F |
Persis |
ACP |
- |
- |
Risk of bleeding |
No issue |
3 months post-procedure |
Worsening exertional dyspnea |
- |
LIPV compression diagnosed on CT and during PVI, mapping at the ridge between LIPV and LAA showed decreased impedance suggesting catheter contact with metal device |
LIPV was compressed with atrial part of ACP |
Successful PVI, ACP was left in place |
Discharged alive |
| Left Circumflex artery Compression |
| Katona et al 2015 |
Hungary |
59 |
M |
Persis |
ACP |
23 |
- |
Recurrent head contusions |
ST elevation in inferior leads |
During procedure |
ST elevation |
LAA with huge ostium |
After positioning of ACP inferior lead showing ST elevation |
Coronary angiogram showing compression of proximal circumflex and device was seen sitting superficially. |
After removal and repositioning of device STE disappeared. |
Discharged |
| Mitral valve impingement |
| Berrebi et al 2017 |
France |
84 |
F |
Pesis |
Amulet |
28 |
5 |
Hemorrhagic shock due to GI bleeding |
No issue. Amulet was in contact with ant. Mitral leaflet but mitral valve kinetics was normal |
6 weeks after procedure |
Denovo mild mitral regurgitation on routine TEE at 6 weeks |
LAA ostium 31 mm and neck 26mm |
tear of A1 portion of anterior mitral leaflet |
Progressive leaflet erosion by amulet outer disc |
Not available |
Not available |
| Cruz-Gonzalez et al 2014 |
Spain |
72 |
F |
Parox |
ACP |
24 |
5 |
GI bleeding |
Pericardial tamponade needing tube drainage after transseptal puncture. Following ACP deployment, inferior part of the external disc appeared over posterior leaflet without any mitral valve dysfunction |
Few days after LAAO |
Recurrent syncope |
LAA neck by angiography 22mm,
-by TEE 19mm
|
Possible dynamic obstruction of valve by device causing syncope |
Compression of posterior mitral leaflet by ACP |
Surgical removal of device and left atrial appendage with resolution of syncope |
Discharged alive |
| Walia et al 2016 |
Taiwan |
61 |
M |
Persis |
ACP |
26 |
5 |
Recurrent strokes and bleeding |
Immediately post-implantation rhythmic movement of ACP disc edge and mild MR were noted |
During the procedure |
Rhythmic movement of disc edge and MR |
Cauliflower morphology
Base 21.8mm
Depth 18.5mm
|
Disc impingement and MR |
Outer disc of ACP causing mitral leaflet impingement |
Removal of device and reimplantation of downsized ACP (24mm) |
Discharged alive |
*all were Caucasian, yr=year,parox=paroxysmal,persis=persistent,LAA=left atrial appendage LAAO=left atrial appendage occlusion, ACP=Amplatzer cardiac plug,mm=millimeter,GI=gastrointestinal, PCI=percutaneous coronary intervention, MI=myocardial infarction, PEA=pulseless electrical activity, STEMI=ST elevation myocardial infarction, LZ=landing zone,MPA=main pulmonary artery,LIPV=left inferior pulmonary vein, PVI=pulmonary vein isolation, MR=mitral regurgitation, STE=ST elevation
Conflict of Interest Disclosures
During the past 12 months Dr. Reiffel has served as an investigator for Janssen, an expert witness for Johnson & Johnson, and a consultant to Roivant. During the past 3 years, Dr. Reiffel has served as an investigator and consultant for Medtronic, Janssen, Gilead, and Sanofi; a consultant for Portola, Acesion, and InCardia Therapeutics; and a member of the speaker’s bureau for Janssen and Boehringer Ingelheim. For this specific manuscript, Dr. Reiffel believes he has no conflict of interest.
Dr. Reiffel is the sole author of this paper, and therefore is responsible for the content and the preparation of the manuscript.
IAB, defined by some as a P wave 0.11 sec or longer and by others as a P wave 0.12 sec or longer: is not uncommon, may take various ECG patterns, may be associated with sinus node dysfunction and its adverse outcomes, may be associated with underlying disorders in which there is an increased incidence of cardiovascular and all-cause mortality, and may be a marker of atrial disease with implications for atrial tachyarrhythmias, such as AF and its complications. Though it commonly is overlooked, IAB should not be missed when evaluating an electrocardiogram.