Apixaban in patients with Atrial Fibrillation: A Systematic Review
Abhishek Maan M.D., E.Kevin Heist M.D., Ph.D., Amir Y. Shaikh M.D., Jeremy N. Ruskin M.D., Moussa Mansour M.D.
Cardiac Arrhythmia Service, Massachusetts General Hospital, 55 Fruit St. GRB 109, Boston, MA 02114.
Atrial fibrillation (AF) is the most common cardiac arrhythmia which increases the risk of stroke and systemic embolism by 5- fold, it is a major global public health problem. Stroke is associated with greatest mortality and morbidity in patients with AF. Strokes associated with AF are especially large and disabling, and consequently primary prevention is paramount. Antithrombotic therapy is the mainstay of stroke prevention. Vitamin K antagonists (VKA's) have been the standard anticoagulants in stroke prophylaxis for patients with AF for decades. Despite their effectiveness, they are limited by several factors such as narrow therapeutic index, drug- drug interactions, slow onset and offset of action, hemorrhage and routine anticoagulation monitoring to maintain therapeutic international normalized ratio (INR). During recent times, various novel anticoagulants have been developed to expand the therapeutic option for stroke prevention. Apixaban is a novel oral anticoagulant which has been developed and clinically investigated for prevention of stroke in AF patients. This review discusses the pharmacological properties, results of clinical trials investigating role of apixaban for prevention of stroke and its future potential in clinical practice.
Correspondence to: Moussa Mansour M.D., Cardiac Arrhythmia service, Massachusetts General Hospital, 55 Fruit St. GRB 109, Boston, MA 02114.
Atrial fibrillation (AF) is a major global public health problem because it is increasing in prevalence and is associated with increased risk of stroke, dementia, heart failure and death.1–4 VKA's have been the mainstay for stroke prevention in AF, their use is limited by various factors such as drug-drug interactions, narrow therapeutic window and the need of lifelong anticoagulation monitoring owing to marked variation from one patient to another and within individual patient.5 An important limitation of Warfarin is the limited time in therapeutic range (TTR) measurable by INR. Randomized control trials have estimated that the INR was in the target range for approximately 36- 68 % .6,7 Aspirin and clopidogrel have also been used as alternative agents to warfarin for stroke prevention. Meta - analysis of six trials have shown that aspirin reduced the risk of stroke by 22 %.8 In the ACTIVE A trial, combination of aspirin and clopidogrel was found to reduce the risk of stroke by 28% with an increased risk of major extracranial and intracranial hemorrhage.9
The ensuing search for safe, effective alternatives with a lower associated risk of bleeding and no need for monitoring and dose adjustment has focused attention on more specific inhibitors of the clotting cascade such as factor Xa inhibitors or direct thrombin inhibitors. The direct thrombin inhibitor dabigatran was approved by the US Food and Drug Administration(FDA) in October 2010 for the prevention of stroke in patients with AF. Rivaroxaban, a factor Xa inhibitor was also recently recommended by an FDA advisory panel as a therapeutic option for the prevention of stroke. This review focuses upon the pharmacological properties and clinical trials and therapeutic potential of apixaban.
We performed a comprehensive literature search in the PubMed database with the keywords; "newer anticoagulants", "atrial fibrillation", "apixaban". Original and clinical studies describing apixaban were included. The language of clinical studies was restricted to English. Previously published reviews describing apixaban were not included as a primary reference source, but were used to identify additional studies of interest.
Apixaban: General aspects and mechanism of action
Apixaban is a reversible, direct and highly selective inhibitor of factor Xa. It has been extensively investigated in pre- clinical studies for the prevention of arterial and venous thrombosis. These studies have demonstrated that apixaban is very efficacious for the prevention of arterial and venous thrombosis at doses which preserve hemostasis.10,11 It has been shown to be a potent inhibitor of both free and cell bound factor Xa and activated prothrombinase.12 These studies have also shown that apixaban causes a rapid inhibition of factor Xa, although it has no direct effects on platelet aggregation, it leads to indirect inhibition of this process by reducing thrombin generation.13
Factor Xa is an attractive target of anticoagulation; it occupies a critical juncture in the coagulation cascade and controls thrombin generation Fig 1. Activation of one molecule of factor Xa leads to generation of 1000 molecules of FIIa.14,15 Although factor Xa inhibition attenuates the production of thrombin, it does not affect thrombin activity, thereby preserving haemostasis, which in clinical terms may translate to lower bleeding risk.16 Compared with thrombin, factor Xa has limited functions outside the coagulation cascade which might contribute to increased efficacy and safety of factor Xa inhibitors.17
Fig 1. Mechanism of action of Apixaban in the coagulation cascade.
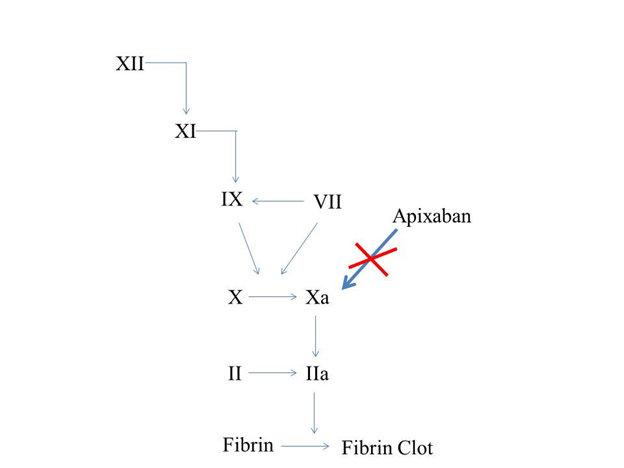
Apixaban is readily bioavailable, pre – clinical studies have demonstrated that it reaches its peak plasma concentration approximately 3 hours after administration, its bioavailability has been estimated to be about 43- 46 % .18 The absorption of apixaban is not affected by food, The various mechanisms of metabolism of apixaban has been demonstrated to be O- demethylation, hydroxylation and sulfation of hydroxylated O- dimethyl apixaban.18,19 Estimated terminal half life of apixaban has been found be 8- 13 hours. Apixaban was not shown to cause significant inhibition or induction of cytochrome P450 enzymes making it relatively less likely to cause drug- drug interactions. Experiments with human cDNA- expressed P450 enzymes and P450 enzyme inhibitors demonstrated that the oxidative metabolism of apixaban was predominantly catalyzed by CYP3A4/5 with a minor contribution of CYP1A2 and CYP2J2, based on these stu dies CYP3A4 and CYP3A5 are expected to play an important role in apixaban metabolism. Apixaban was also metabolized by human intestinal microsomes and not by kidney microsomes suggesting that liver and intestines are the major organs involved in its metabolism.19 Pre- clinical studies have demonstrated that apixaban has multiple elimination pathways. Apixaban is renally excreted to an extent of 25 %, fecal (55 %) and biliary excretion contribute as other mechanisms for excretion.18–20 Multiple elimination pathways suggest that apixaban could be a superior therapeutic option in patients with hepatic or renal impairment. Dabigatran, which was approved by the FDA for prevention of stroke in patients with AF is renally excreted upto the extent of 80 %,21 Rivaroxaban which was also recently recommended by the FDA as an alternative anticoagulant in patients with AF has a dual mode of elimination with one – third of the unchanged active drug eliminated renally; the remaining two- thirds of the drug are eliminated by the liver.22 Compared with the other two newer anticoagulants, apixaban is a relatively safer option in patients with renal insufficiency owing to its multiple pathways of elimination. Unlike dabigatran, its absorption is not affected by the presence of food.23Table 1.
Table 1. Comparison of Apixaban with Dabigatran and Rivaroxaban
| Parameter |
Apixaban |
Dabigatran |
Rivaroxaban |
| Target |
Factor Xa |
Thrombin |
Factor Xa |
| Prodrug |
No |
Yes |
No |
| Oral Bioavailability |
43- 46 % |
6.5 % |
80 % |
| Dosing Frequency |
Fixed, twice daily |
Once or twice daily |
Once or twice daily |
| T (max) |
3 hrs |
1-2 hrs |
2.5 – 4 hrs |
| Half – life |
8- 13 hrs |
12-14 hrs |
7- 13 hrs |
| Routine coagulation Monitoring |
No |
No |
No |
| Elimination |
25 % renal, 75 % fecal and biliary |
80 % Renal |
67 % renal , 33 % fecal |
| Potential drug interactions |
Potent CY3PA4 |
Rifampin, Amiodarone, Potent P-gp |
Potent inhibitors of CYP3A4 and P-gp |
| Clinical Status |
Awaits FDA review |
Approved for stroke prevention in Oct 2010 |
Recommened by FDA advisory panel for future use for stroke prevention in Sept 2011 |
1. T (max) : Peak plasma levels, 2. CYP: Cytochrome P450, potent CY3PA4 inhibitors include azole antifungals, macrolide antibiotics (e.g. clarithromycin), and protease inhibitors (atazanavir), 3. P-gp: P- glycoprotein, potent CY3PA4 and P-gp inhibitors include azole antifungals (e.g. ketoconazole, itraconazole) and protease inhibitors such as ritonavir.
Table 2. Comparison of clinical trials of Apixaban for stroke prevention
| Trial Name |
AVERROES |
ARISTOTLE |
| Comparison arms |
Apixaban vs. Aspirin |
Apixaban vs. Warfarin |
| Study dose of apixaban |
5 mg bid or 2.5 mg bid † |
5 mg bid or 2.5 mg bid † |
| Number of patients |
5599 |
18,201 |
| Patient age (years ) |
Mean: 70 + 9 |
Median: 70 |
| Mean CHADS2 Score |
2.0 + 1.1 |
2.1 + 1.1 |
| Hazard ratio with 95 %confidence interval of 1º outcome(stroke and systemic embolism) |
0.45, 0.32 to 0.62; p <0.001 |
0.79, 0.66 to 0.95; p <0.001
for noninferiority, p= 0.01 for superiority
|
| Hazard ratio with 95 % confidence interval for all- cause mortality |
0.79, 0.62 to 1.02; p = 0.07 |
0.89, 0.80 to 0.99; p = 0.047 |
| Hazard ratio with 95 % confidence interval for major bleeding |
1.13, 0.74 to 1.75; p = 0.57 |
0.69, 0.60 to 0.80; p<0.001 |
| Hazard ratio with 95 % confidence interval for non major bleeding |
1.15,0.86 to 1.54; p = 0.35 |
0.68, 0.61 to 0.75; p <0.001 |
†: Reduced dose in patients with two of the following criteria (age > 80 years, body weight < 60 kg, or a serum creatinine of > 1.5 mg/dl
Table 3. Comparison of main clinical trials of the newer anticoagulants vs. Warfarin for prevention of thromboembolism in atrial fibrillation
| Drug Name |
Apixaban |
Dabigatran |
Rivaroxaban |
| Drug Class |
Factor Xa inhibitor |
Thrombin Inhibitor |
Factor Xa inhibitor |
| Trial Name |
ARISTOTLE |
RE-LY |
ROCKET – AF |
| Number of patients |
18,201 |
18,113 |
14,264 |
| Dose of study drug |
5 mg bid, or 2.5 mg bid* |
150 mg bid, or 110 mg bid † |
20 mg daily, or 15 mg dailyl |
| Patient age (years) |
Median: 70 |
Mean: 71.4 + 8.6 |
Median: 70 |
| Mean CHADS2 score |
2.1 + 1.1 |
2.1 + 1.1 |
3.48 + 0.94 |
| Mean time in therapeutic range for Warfarin |
62 % |
64 % |
55 % |
| Hazard Ratio with 95% confidence interval of 1º outcome (stroke and systemic embolism) |
0.79; (0.66-0.95)
P < 0.01 |
0.66; (0.53-0.82)
P<0.001 for 150mg dose, 0.91; (0.74-1.11)
P=0.34 for 110 mg dose |
0.88; (0.74-1.03)
P=0.12 |
| Hazard ratio with 95% confidence interval for all – cause mortality |
0.89; (0.80-0.99)
P = 0.047 |
0.88;(0.77-1.00)
P = 0.051 for 150 mg dose
0.91; (0.80-1.03)
P = 0.13 for 110 mg dose |
0.92; (0.82-1.03)
P = 0.15 |
| Hazard ratio with 95% confidence interval for major bleeding |
0.69; (0.60-0.80)
P <0.001 |
1.03;(0.96- 1.11)
P=0.44 |
0.93; ( 0.81 -1.07);
P=0.31 for 150 mg dose,
0.80;(0.69-0.93) for 110mg |
| Hazard ratio with 95% confidence interval interval for major bleeding |
0.68;(0.61-0.75)
P<0.001 |
0.91;(0.85-0.97)
P<0.005 for 150 mg dose
0.79;(0.74-0.84)
P<0.001 for 110mg dose |
1.04; (0.96-1.13)
P=0.35 |
* Two different drug dosages were tested in the study, † Reduced dose used in patients with creatinine clearance of 30-49 ml/minute, l Reduced dose in patients with two of the following criteria (age > 80 years, body weight < 60 kg, or a serum creatinine of > 1.5 mg/dl
Clinical Trials of Apixaban for stroke prevention in AF
The AVERROES trial was the first major clinical study published in February 2011 which investigated the efficacy of apixaban compared with aspirin for stroke prevention in patients with AF. It was a randomized, multicenter, double – blind clinical trial which included 5599 patients with AF who were at increased risk of stroke and for whom VKA therapy was considered unsuitable. In the apixaban arm majority of the patients received a dose of 5 mg twice daily. The dose of apixaban was reduced to 2.5 mg twice daily in about 6% of the patients in apixaban arm (patients > 80 years of age, body weight < 60 kg, or serum creatinine of > 1.5mg /dl). In the comparison arm, aspirin was used in the dose of 81- 324 mg. The mean age of the patients participated in both arms of the study was 70 +/- 9 years, mean CHADS2 score of the patients in the study was 2.0 + 1.1, the patients in both arms were followed for average period of 1.1 years. The primary outcome of the trial was the occurrence of stroke or systemic embolism, secondary outcomes measured in the study were overall mortality, hospitalization due to cardiovascular causes. The primary safety outcome of the study was major bleeding, defined as clinically overt bleeding accompanied by one of more of the following; a decrease in the level of hemoglobin of 2 g per deciliter or more over a period of 24 hours, bleeding requiring transfusion of 2 or more units of packed red blood cells, any bleeding at critical sites (intracranial, intraspinal, intraocular, intraarticular, intraocular, pericardial, intramuscular with compartment syndrome, or, retroperitoneal) or fatal bleeding.
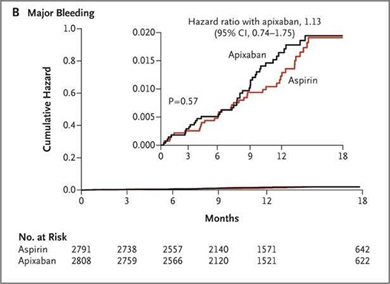
In this study, apixaban was found to significantly reduce stroke or systemic embolism as compared to aspirin (1.6 % per year in apixaban arm vs. 3.7 % per year in aspirin arm, hazard ratio with apixaban, 0.45; 95 % confidence interval [CI] 0.32 to 0.62; p <0.001). Apixaban was not found to cause significant reduction in overall mortality as compared to aspirin (3.5 % per year in apixaban arm vs. 4.4 % per year in the aspirin arm, hazard ratio, 0.79; 95 % CI, 0.62 to 1.02 ; p = 0.07) Fig 2. Apixaban was not found to have increased risk of bleeding as compared to aspirin in this study. There were 44 cases of major bleeding (1.4 % per year) in apixaban arm vs. 39 cases (1.2 % per year) in the aspirin arm (hazard ratio with apixaban, 1.13; 95 % CI, 0.74 to 1.75; p = 0.57) Fig 3.24
Fig 2 Cumulative Hazard rates for the Primary efficacy outcomes (stroke and systemic embolism) for apixaban vs. aspirin in the AVERROES trial (reproduced with permission from New England Journal of Medicine)
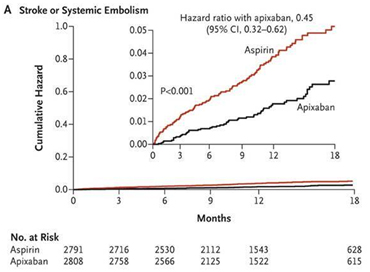
Fig 3 Cumulative Hazard rates for the Primary safety outcomes (Major bleeding) for apixaban vs. aspirin in the AVERROES trial (reproduced with permission from New England Journal of Medicine)
The ARISTOTLE trial was another recently published major clinical trial which investigated the efficacy of apixaban compared with warfarin for prevention of stroke in patients with AF. It was a randomized, multicenter, double – blind clinical trial which included 18,201 patients with AF who had at least one additional risk factor for stroke. In the apixaban arm, majority of the patients received a 5mg bid dose. A reduced dose of apixaban (2.5 mg bid) was used in patients with 4.7 % of the patients with at least 2 of the following criteria (age > 80 years of age, body weight < 60 kg, or a serum creatinine of > 1.5mg /dl). In the comparison arm the dose of warfarin was adjusted according to INR. The median age of the patients in the trial was 70 years, with a mean CHADS2 score of 2.1, patients in the clinical trial were followed for a median duration of 1.8 years. The primary efficacy outcome was ischemic or hemorrhagic stroke or systemic embolism. This trial was designed to test for non inferiority, with key secondary objective of testing for superiority with respect to the primary outcome. The key secondary efficacy outcome of the study was death from any cause and myocardial infarction. Primary safety outcome of the study was major bleeding (definition same as that in AVERROES trial), secondary safety outcome of the study was a composite of major bleeding and clinically relevant non major bleeding.
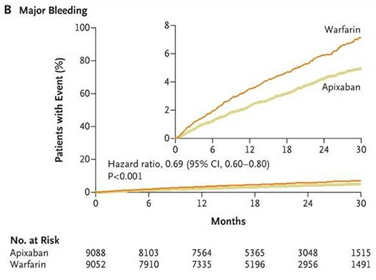
In this study, apixaban was found to be superior to warfarin for prevention of stroke and systemic embolism. The rates of primary efficacy outcome was 1.27 % per year in apixaban arm, as compared with 1.60 % per year in the warfarin arm (hazard ratio with apixaban, 0.79; 95 % CI, 0.66 to 0.95; P <0.001 for non inferiority; P = 0.01 for superiority) Fig 4. A highlight of the study was significant reduction in overall mortality in the apixaban arm (3.52 % in the apixaban group vs. 3.94 % in the warfarin arm, hazard ratio, 0.89; 95 % CI, 0.80 to 0.99; P = 0.047). Apixaban was also found to cause significantly lesser bleeding as compared to warfarin (2.13 % per year in apixaban arm vs. 3.09 % per year in the warfarin arm, hazard ratio , 0.69 ; 95 % CI, 0.60 to 0.80; P <0.001)Fig 5.25
Fig 4 Cumulative Hazard rates for the Primary efficacy outcomes (stroke and systemic embolism) for apixaban vs. warfarin in the ARISTOTLE trial (reproduced with permission from New England Journal of Medicine)
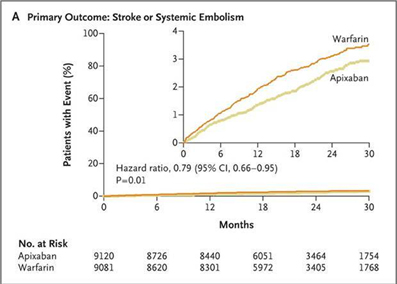
Fig 5 Cumulative Hazard rates for the Primary safety outcomes (Major bleeding) for apixaban vs. warfarin in the ARISTOTLE trial (reproduced with permission from New England Journal of Medicine)
Following the successful clinical outcomes of apixaban in the AVERROES and ARISTOTLE trial, it appears to be a promising novel anticoagulant for prevention of stroke in patients with AF. In the various major subgroups of the patients, reduction in primary efficacy outcomes was consistently maintained. Apixaban also seems to have an acceptable side- effect profile which is evident by lower rate of discontinuation as compared with warfarin. Apixaban not only is more effective than warfarin for stroke prevention but also accomplishes the goal at a substantially lower risk of bleeding.
Currently there are two alternative oral anticoagulants to warfarin, the direct thrombin inhibitor dabigatran26 and the factor Xa inhibitor rivaroxaban.27 Rivaroxaban was recently recommended by an FDA advisory committee as a future therapeutic option for stroke prevention in patients with AF. Each of these agents, like apixaban has the major advantage of convenience, since they do not require anticoagulation monitoring.
Dabigatran was evaluated in the Randomized Evaluation of Long- Term Anticoagulation Therapy trial (RE-LY), in this trial, the 150 mg twice daily dose was observed to reduce the rate of stroke and systemic embolism with a similar overall rate of bleeding, it is however worth mentioning that dabigatran was associated with higher rate of gastrointestinal bleeding.28 Dabigatran was also found to be associated with higher rate of dyspepsia (11.8 % of the patients in 110 mg dose group, 11.3 % of the patients with 150 mg dose group) as compared with warfarin (5.8% of the patients). This might be attributable to the formulation of dabigatran which contains a tartaric acid moiety core to enhance intestinal absorption (the optimal bioavailability requires a low pH), which could also account for higher rate of gastrointestinal bleeding with dabigatran29,30 As compared with warfarin, apixaban was found to have lower rates of gastrointestinal bleeding and with consistently lower rates of bleeding across age groups. Renal excretion of apixaban is estimated to be approximately 25% as compared to 80 % for dabigatran,30,31 which suggests that apixaban could be a relatively safer anticoagulant in patients with renal impairment.
Rivaroxaban is the second alternative to warfarin which was recently recommended by an FDA advisory panel as a future therapeutic option for stroke prevention in patients with AF. In the ROCKET AF trial, it was shown to be noninferior to warfarin for the prevention of stroke and systemic embolism. The rates of intracranial hemorrhage and fatal bleeding were observed to be lower with rivaroxaban, but there was no advantage with respect to other major bleeding.27
Compared with the clinical outcomes of dabigatran and rivaroxaban, apixaban seems to offer an additional advantage of significantly reducing all – cause mortality as compared with warfarin, as observed in the ARISTOTLE trial.
Development of apixaban and other novel anticoagulants have necessitated the need for development of monitoring options so that their effect and patient compliance can be monitored. Currently there are no standardized monitoring tests available for any of the newer anticoagulant. Pre-clinical studies have shown that modified prothrombin time (m PT) and Hep Test seem to have the highest sensitivity to apixaban, and seem to be promising coagulation assays to monitor the anticoagulant activity of apixaban, but further clinical validation is needed.8,32
Another important potential problem which will need to be addressed in the near future is the reversibility of novel anticoagulants in clinical situations which require immediate reconstitution of coagulation system (e.g. severe bleeding events). There are currently no recommendations or available antidotes to reverse the action of direct thrombin inhibitors or factor Xa inhibitors. Recombinant factor VIIa (NovoSevenR) and activated prothrombin complex concentrate (FEIBAR) have been discussed to reverse the effect of direct factor Xa inhibitors, but there are no sufficient data to routinely recommend their clinical use for this purpose.33,34,35
The short half – life of apixaban may help to prevent clinically relevant bleeding events (which are observed in over- anticoagulated patients treated with oral VKA) but also requires a good patient compliance, because missing 2-3 doses can lead to ineffective anticoagulation. Development of new coagulation assays will also be helpful in monitoring patient compliance to new anticoagulants.
Apixaban seems to be a promising novel anticoagulant as evident by recently published clinical studies for stroke prevention in patients with AF. Further results of ongoing clinical trials with apixaban will establish its safety before practitioners make fundamental changes in their clinical practice. Along with two other novel anticoagulants, dabigatran and rivaroxaban, apixaban may replace warfarin as the preferred anticoagulant for stroke prevention in patients with AF.
Biosense Webster Consultant, Research Grant
Boston Scientific Consultant, Research Grant
St. Jude Medical Consultant, Research Grant
Medtronic Consultant
MC 10 Research Grant
Voyage Medical Research Grant
Rhythmia Medical Research Grant
Cardiofocus Research Grant