Leadless Pacemakers – Implant, Explant and Long-Term Safety and Efficacy Data
Krishna Kancharla, Abhishek J Deshmukh, Paul A Friedman
Division of Cardiovascular Diseases, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Implantable cardiac pacemakers have seen remarkable progress in the last sixty years and remained as cornerstone therapy for symptomatic bradycardia. Despite this progress the current day traditional transvenous implanted pacemaker systems are limited by the need for a surgically created pocket for the generator, indwelling leads in the vascular system and lastly passage through the tricuspid valve. A majority of the implant and explant related complications are due to the surgical pocket and indwelling leads. Leadless pacemakers represent a major leap in technology and emerged as an alternative to traditional systems promises to eliminate lead and pocket associated complications. As with any disruptive technology, some questions remain unanswered with the leadless pacing systems, specifically longevity and end of life management for the device. Despite the unknowns, as the technology progresses, it is possible that pacing leads will become extinct and pacemakers will miniaturize even further. This review summarizes the available technology, implant and explant details, and long-term safety and efficacy data for leadless pacemakers.
Key Words : Leadless pacemaker, Cardiac device, Percutaneous delivery, Cardiac implant, pacemaker.
Correspondence to: Paul A Friedman, MD Professor of Medicine Department of Cardiovascular Diseases Mayo Clinic College of Medicine 200 First Street SW Rochester, MN 55905 Phone: 507-255-2446 Fax: 507-255-2550 E-mail: friedman.paul@mayo.edu
“When Henry Ford made cheap, reliable cars people said, 'Nah, what's wrong with a horse?' That was a huge bet he made, and it worked” - Elon Musk
Initial interest for cardiac pacing was reported in the 1930s with Hyman’s “artificial pacemaker” (his term), in which a hand crank created an electric current that drove a DC generator directing electrical impulses to the patient’s right atrium through a needle electrode placed through intercostal space. At that time, due to perceived disruptive nature of this approach Hyman faced professional skepticism, litigation, and accusations of creating “an infernal machine that interferes with the will of God”.[1]
Cardiac implantable electrical devices have seen remarkable progress in the last sixty years. Since the first entirely implantable pacemaker performed in 1958, major advancements in design, complexity and battery longevity made implantable pacemaker therapy a very acceptable option and have benefited millions of patients around the world. Despite these improvements the current day traditional transvenous implanted pacemaker systems are limited by the need for a surgically created pocket for the generator, indwelling leads in the vascular system and lastly passage through the tricuspid valve. The pocket is associated with risk of hematoma and infection with initial implant and every generator replacement. The intravascular leads pose a risk of access site complications, venous thrombosis and occlusion, lead malfunction, and infection often necessitating entire system extraction. [2], [3] Passage of right ventricular lead through the tricuspid valve is associated with risk of valve dysfunction and regurgitation that may result in symptomatic right heart failure requiring repair.[4] The leads are inherently thrombogenic, eliciting fibrotic reactions that make removal technically challenging, with a risk of venous perforation, valve disruption, hemothorax, and death.[5] The leadless pacing system was developed to overcome these limitations, and represents a major leap in technology that allows a completely intracardiac implant. The introduction of “leadless” pacing systems as an alternative to traditional systems promises to eliminate lead and pocket associated complications.
To date, two leadless pacing systems have been introduced. The NanostimTM leadless cardiac pacemaker-LCP [Figure 1] (St. Jude Medical, Sylmar, CA, USA) (currently on voluntary hold due to a battery advisory) and the MicraTM transcatheter pacing system –TPS (Medtronic, Minneapolis, MN, USA)[Figure 1].
Figure 1. The NanostimTM leadless cardiac pacemaker -LCP (St. Jude Medical, Sylmar, CA, USA) on the left image. Modified with permission from Abott/ St. Jude. MicraTM transcatheter pacing system – TPS (Medtronic, Minneapolis, MN, USA) on the right of the image. Modified with permission from Medtronic, Minneapolis, MN, USA.

Both devices are completely contained single units with battery and circuit material contained in a small metallic unit with bipolar sensing and pacing electrodes. Each of these devices has a fixation mechanism mounted at the distal end and a docking mechanism to deliver and retrieve the device built at the proximal end [Figure 1]. A steroid eluting electrode is present at the distal end independent of the fixation components. Comparative details on the devices are listed in [Table 1]. LCP utilizes a screw-in helix based active fixation and a secondary fixation mechanism with tines compared to TPS which used selfexpanding nitinol tines. LCP has a high-density lithium carbon mono fluoride battery with 248 mAh energy capacity compared to TPS which uses Lithium silver vanadium oxide/carbon mono fluoride with 120 mAh energy capacity. TPS has capture management feature to support battery efficiency. Both devices operate in a single chamber mode (VVI/VVIR) and have rate response feature, LCP utilizing blood temperature and TPS using a 3 – axis accelerometer. LCP uses conductive communication through the skin leads connected to the programmer, using 250 KHz sub-threshold pulses encoded with data, delivered during the ventricular refractory period. This supports energy saving compared to a standard radiofrequency communication used in TPS system and traditional pacemakers.
Table 1. Leadless pacemaker device comparison. [13], [14]
| Parameter |
Nanostim (LCP) |
Micra (TPS) |
| Length (mm) |
42 |
25.9 |
| Diameter (mm) |
5.99 |
6.7 |
| Volume (cm3) |
1 |
0.8 |
| Weight (gram) |
2 |
2 |
| Power source |
high-density lithium carbon monofluoride battery |
Lithium silver vanadium oxide/carbon monofluoride |
| Energy capacity |
248 |
120 |
| Battery longevity(years) |
9.8
100%/2.5 V/0.4 msec/60b.p.m. |
10
100%/1.5 V/0.24 msec/60 b.p.m. |
| Delivery sheath size |
18F |
27F |
| Fixation |
Screw-in helix and 3 nitinol tines |
Four self-expanding nitinol tines |
| Pacing Mode |
VVI/VVIR |
VVI/VVIR |
| Rate response |
Blood temperature |
Programmable 3-axis
Accelerometer |
| Communication/ Telemetry |
Conductive communication |
Radiofrequency |
| Capture management |
No |
Yes |
Currently, available leadless devices design only allows single chamber operation with VVI/ VVIR modes. The most common indication is atrial fibrillation with the atrioventricular (AV) block. Other indications are sinus bradycardia with infrequent pauses and sinus rhythm with AV block in patients with low level of physical activity or shortened life span. In addition, for patients with a history of prior device infections and poor subclavian vascular access leadless pacing becomes attractive option compared to transvenous implantation. For young patients with and infrequent but compelling pacing indication, a leadless pacemaker may be considered, recognizing that optimal management at the time of battery depletion (extraction vs. abandonment) is not known.
Preparation prior to implantation
Prior to implantation it is important to confirm no contraindications exist for device implantation such as the presence of an interrupted IVC, IVC filter, mechanical tricuspid valve, morbidly obese that could lead to difficulty in communication with the device, intolerance to Nickel-Titanium (Nitinol) Alloy, allergy to dexamethasone acetate, another implanted device that can interfere with functioning of the device (e.g. Neurostimulator), other intracardiac implants/ leads that could interfere with the leadless system.
Pre-procedural review of anesthesia, anticoagulation strategy for the procedure, ability to use contrast, fluoroscopy or ultrasound for visualization should be considered. Implant equipment including access sheath, deflectable delivery unit, and multiple flush lines should be confirmed. The strategy for hemostasis (manual compression versus suture-based closure) should be planned and necessary tools are available in advance.
Both LCP and TPS are implanted via femoral venous access and catheter-based delivery system under fluoroscopic guidance. With a deflectable delivery sheath [Figure 2]-[Figure 3] the docked unit is advanced from inferior vena cava to right atrium and then through the tricuspid valve into the right ventricle. Typically angiogram of the RV is performed to select the target site of implantation. LCP is implanted by the rotating the screw-in helix with the help of the chevron (marker) on the fluoroscopy for 1.25 turns. TPS has self-expanding nitinol tines that deploy after retracting the outer sheath over the device (Video 1). After fixation, the device is undocked for testing the sensitivity and capture threshold. If adequate functioning is not achieved then the device can be repositioned. After an adequate positioning, a tug test (video 2) is performed to assess the stability of the device and the released from the delivery system.
Figure 2. LCP delivery system. A shows delivery catheter system with the LCP at the distal end. The proximal handle has the features to adjust the deflection of the catheter and release the device after fixation. B shows LCP docked with the catheter. C shows LCP undocked, but tethered during which tug test can be performed to assess stability. Modified with permission from Abott/ St. Jude.
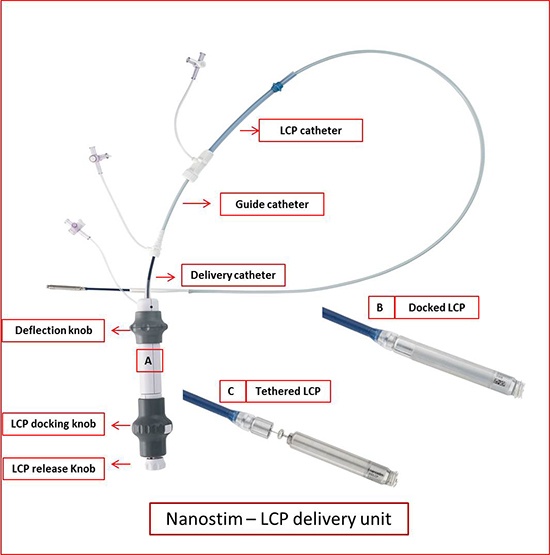
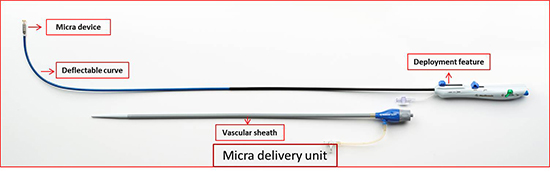
Figure 3.
Post-implantation monitoring
Typically patients are monitored overnight for vital signs, access sites, hemostasis and telemetry monitoring. An EKG and telemetry serve for arrhythmia monitoring. Chest x-ray in two views can help confirm the stability of the device. A device interrogation post implant to confirm continued adequate functioning is appropriate. Patients will need access site care and restriction on activity until adequate healing.
The benefit of explantation of leadless pacemaker should be weighed against risks associated with the procedure. Having experience with leadless pacemaker implantation and intravenous device explantation can help during the explantation of leadless system. Common reasons for removal of the device are elevated pacing threshold, change in pacing indication to a cardiac resynchronization or dual chamber pacing.[6 Other possible indications include frequent premature ventricular complexes or arrhythmia thought to be related to the device, infection and dislodgement/ migration.
A study [7] involving nine centers and 16 patients with LCP devices retrieved and showed 94% success rate in explantation without any 30-day complications. All 5/5 patients who had acute retrieval (< 6 weeks) had successful explant and 10/11 (91%) patients who had chronic retrieval (≥ 6 weeks (range, 88–1188 days) had successful explant. The indication for device removal in the acute retrieval group was an elevation in pacing threshold in 4 patients and need for an upgrade to a secondary prevention defibrillator in one patient. In chronic retrieval group, the indications included elevation in pacing threshold in 4/11 patients, right ventricular pacing-induced cardiomyopathy in 5/11, failure to pace in 1/11, and patient preference in 1/11. Seven of the 11 patients had a new leadless device implanted. Experience with TPS retrieval is limited. After the initial day of implantation, there were six percutaneous attempts at removal listed [8]of which four were noted to be successful. A controlled report of the data on TPS retrieval is not available at this time.
Preparation prior to explanation
Prior to extraction of a leadless device, anticoagulation should be withheld and reversed if appropriate. In patients who are dependent upon pacing, alternative pacing support should be achieved either via a temporary or permanent means. Echocardiography (Intracardiac or transthoracic) can aid in retrieval process and also in the evaluation of pericardial effusion. An arterial access for adequate hemodynamic monitoring is reasonable. Retrieval of a leadless system requires femoral venous access, a deflectable retrieval sheath and snaring tools [Figure 4]. A Single- and tri loop retrieval catheters designed specifically for chronic LCP retrieval were available and can be utilized. TPS recommends standard retrieval tools (e.g. Amplatz Gooseneck® Snare Kit) that are available off the shelf. A plan to deal with pericardial effusion/tamponade should be in place prior to extraction. Depending upon the extent of injury the intervention can range from percutaneous pericardial drainage to surgical repair.
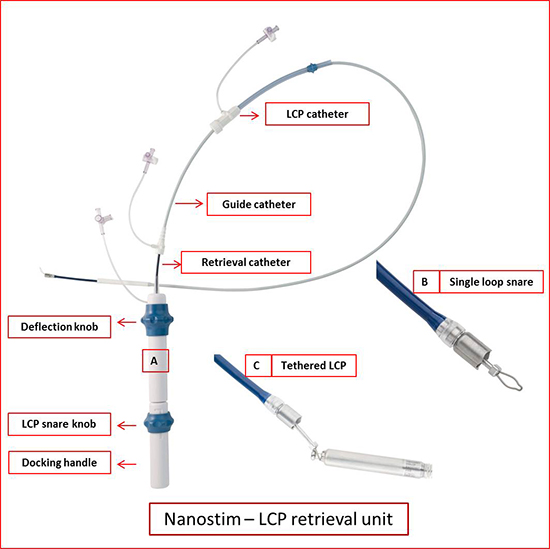
Figure 4. LCP retrieval system. A shows retrieval catheter system with single loop snare at the distal end. The proximal handle has the features to adjust the deflection of the catheter and snare the device and dock it. B shows a single loop snare. C shows LCP tethered with the snare. Modified with permission from Abott/ St. Jude.
A femoral venous access is obtained followed by insertion of the vascular sheath. A deflectable vascular sheath is inserted and advanced into the right atrium and then through the tricuspid valve into the right ventricle. Both devices have a knob-like structure on the proximal end that can be snared with the use of a snare. Once the LCP is docked it can then be rotated to unscrew the helix from the endocardium [Figure 5]. TPS has tine based fixation and thus once snared gentle pull in the axis of the device is performed to assess for removal. Once free the devices should be pulled back into the protective sleeve for removal from vascular space. Intracardiac imaging can be valuable in guiding extraction and assessment of effusion. Once extracted the device should be assessed for complete removal [Figure 6].
Figure 5. LCP retrieval. Figure A-D shows snare tool over the device to capture the docking knob. Figure E shows advancing the docking tool to fix with the device. Figure F shows deflectable sheath advanced over the device. Figure G shows unscrewing the device. Figure H-I show removal of the entire system for the right ventricle.
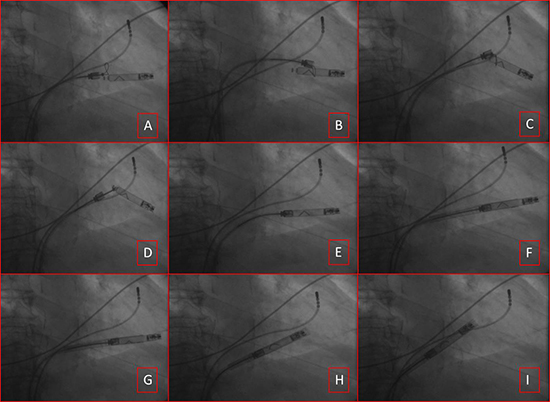
Figure 6. Explanted LCP device. Left side image shows docked explanted device. Right image shows snared device with docking tool removed.
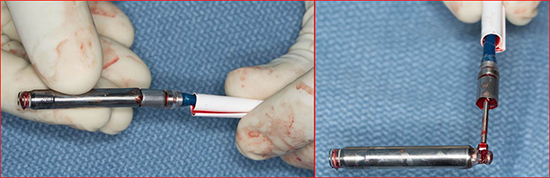
Post explantation monitoring
Post explantation monitoring is similar to standard device explantation. Due to the vascular access patients will need 2-4 hours bed rest after hemostasis. Overnight monitoring for access site complications, vital signs, and telemetry for arrhythmia are appropriate. A post procedure x-ray to confirm all device components have been removed and evaluation for effusion is appropriate. Patients will need access site care and restriction on activity until adequate healing.
LCP system: The LEADLESS II trial [9], a prospective multicenter study, provided the initial safety and efficacy data for LCP device. The study used historical control group as a comparison. The primary efficacy end point was an acceptable pacing threshold (≤2.0 V at 0.4 msec) and acceptable sensing amplitude (R wave ≥5.0 mV, or a value equal to or greater than the value at implantation). The primary safety end point was freedom from device-related serious adverse events. Through a six month period primary safety endpoint was met in 270 of the 300 patients in the primary cohort (90.0%) and exceeded the pre-specified performance goal of 85% [Table 2]. The primary safety endpoint was met in 280 of the 300 patients at six months (93.3%) and exceeded the pre-specified goal of 86%. In the total cohort of 526 patients, device-related serious adverse events occurred in 6.5%, including cardiac perforation in 1.5% of the patients, device dislodgement in 1.1%, and device retrieval due to elevated pacing thresholds in 0.8%. All patients with device dislodgements had percutaneous retrieval. There were two deaths (0.4%) that were classified by the clinical events committee as procedure related [Table 3].
Table 2. Leadless pacemaker implantation – Efficacy outcomes [9], [11]
|
Nanostim (LCP) |
Micra (TPS) |
| Implant success(%) |
95.8 |
99.2 |
| Pacing capture Threshold and sensing efficacy endpoint (%) |
90.0 (≤2.0 V at a pulse width of 0.24 msec and an increase of ≤1.5 V
from the time of implantation) |
98.3 (≤2.0 V at 0.4 msec and
sensing amplitude ≥5.0 mV,
or a value ≥ value at
implantation) |
| Threshold at implant |
0.82 V @ 0.4 |
0.63 V @ 0.24 |
| Threshold at 6 months |
0.4 V @ 0.4 |
0.54 V @ 0.24 |
| Sensing at 6 months |
10.6 mV |
15.3 mV |
Table 3. Leadless pacemaker implantation – Safety outcomes [9], [11]
|
Nanostim (N=526)(%) |
Micra (n=729) (%) |
| Total events |
6.5 |
4 |
| Cardiac perforation |
1.6 |
1.6 |
| Vascular complication |
1.2 |
0.7 |
| Arrhythmia during device implantation |
0.6 |
0 |
| Cardiopulmonary arrest during implantation procedure |
0.2 |
0 |
| Device dislodgement |
1.1 |
0 |
| Device migration during implantation owing to inadequate fixation |
0.4 |
0 |
| Pacing threshold elevation with retrieval and implantation of new device |
0.8 |
0.3 |
| Pulmonary embolism |
0.2 |
0.1 |
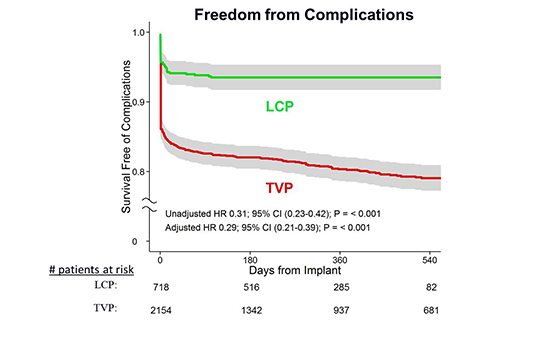
At one year follow-up of LEADLESS trial [10], there were no additional pacemaker-related adverse events reported. The mean pacing threshold at 6- and 12-month follow-up were, 0.40V and 0.43V at 0.4msec. R-wave amplitudes were 10.6mV and 10.3mV respectively. At the 12-month follow-up an adequate rate response was observed in all patients in whom it was activated. In comparison to matched controls, patients who received LCP device had a significantly improved freedom from complications (HR = 0.29 (0.21-0.39), p<0.001) [Figure 7]. Currently, the device implantation is on a voluntary hold from the company in a review of lost telemetry and pacing output in a very small proportion (<0.5%) of patients. No clinical consequences were reported due to this at present.
Figure 7. Freedom from complications from LCP device in comparison with matched controls who received transvenous pacing devices. Permissions pending.
TPS system: Micra TPS study [11], a prospective multicenter study, provided the initial safety and efficacy data for TPS device. The primary efficacy end point was percentage of patients with low and stable pacing capture thresholds at 6 months (≤2.0 V at a pulse width of 0.24 msec and an increase of ≤1.5 V from the time of implantation). The primary safety end point was freedom from system-related or procedure related major complications. The primary efficacy end point was met in 292 of 297 patients in the primary cohort at six months (98.3%) and exceeded the prespecified performance goal of 80%. [Table 2] The primary safety endpoint was met in 96% patients and exceeded the prespecified goal of 83%.[8 In the total cohort of 725 patients,". device-related serious adverse events occurred in 4%, including cardiac perforation in 1.6% of the patients, device dislodgement in none and device retrieval due to elevated pacing thresholds in 0.3% patients.
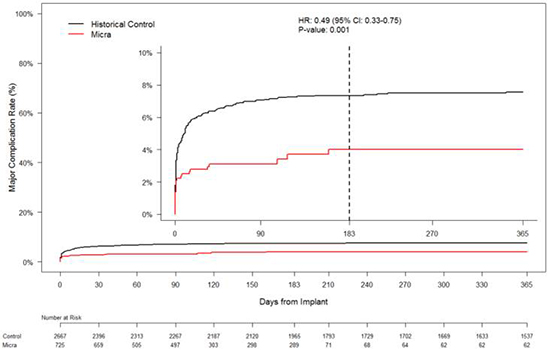
At one year follow up adequate device sensing and capture [Figure 8] was reported without any adverse events. In comparison to the historical controls who received transvenous devices, the study patients were older with more comorbidities. The Micra group had 51% fewer major complications, 54% fewer hospitalizations and 87% fewer system revisions (0.4% vs. 3.5%) due to complications compared to historic controls. [Figure 9] There are no reported cases of leadless system infections to date.[12]
Figure 8. Electrical Performance Characteristics of the Transcatheter Pacemaker (TPS). Permissions pending.
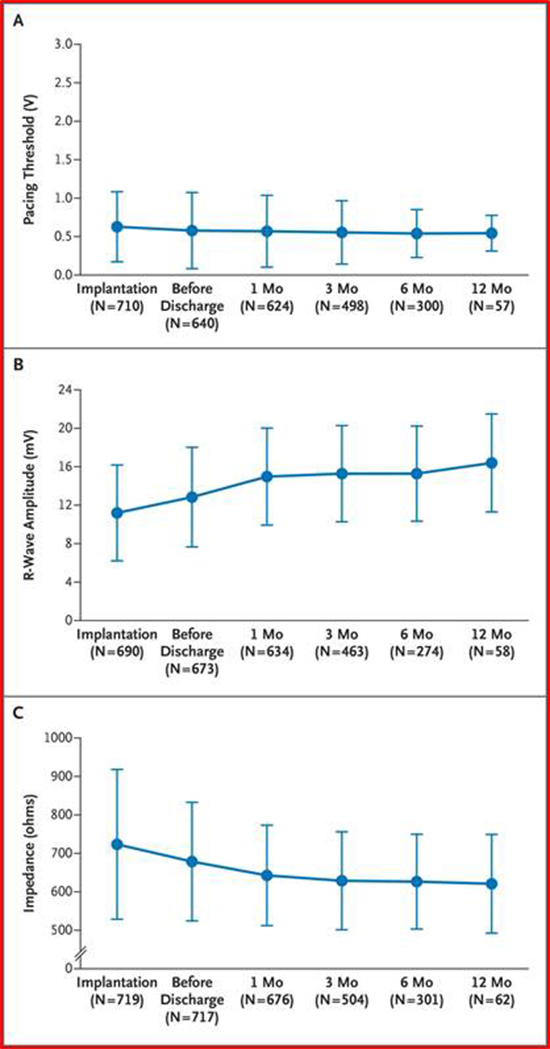
Figure 9. Major complications of Transcatheter Pacemaker (TPS) system in comparison with historical cohort who received transvenous pacing devices. Permissions pending.
Currently available leadless device technology only allows single chamber operation and thus limits therapy only to a minority of patients. In patients with sinus rhythm requiring atrioventricular synchrony, dual chamber device therapy is more appropriate. Feasibility of multi-chamber operation with leadless pacemakers is yet to be seen. Utility of leadless pacemakers along with subcutaneous ICD and cardiac resynchronization therapy also needs to be studied.
The efficacy and safety seem acceptable in the midterm follow-up with leadless systems. The long-term effectiveness and overall battery longevity data in comparison to traditional pacemakers is currently not available and remains to be evaluated.
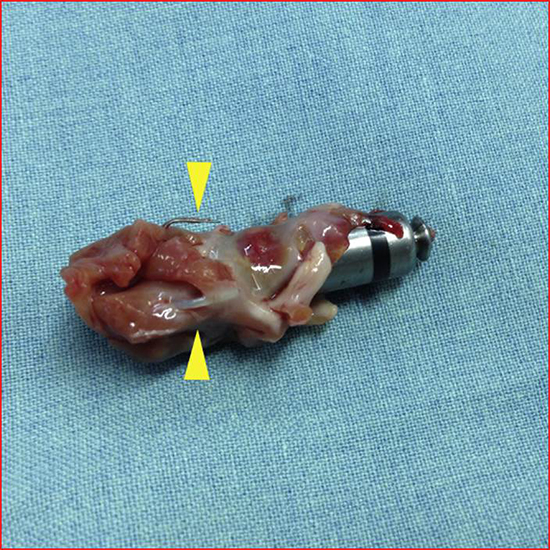
There is limited data regarding retrievability of the leadless devices at this time. The encapsulation and tissue growth over the device can result in challenges for extraction [Figure 10]. Feasibility and safety data on extraction of long-term chronic implants is not known. If devices are not retrievable, then the feasibility of co-implantation of additional pacemaker system/ systems and effect on cardiac function need to be studied.
Figure 10. Autopsy specimen in a patient who received Micra device showing tissue growth and tines densely adhered to tissue. Permissions pending.
As with any disruptive technology, a number of questions remain unanswered with the leadless pacing systems. Randomized clinical trials will be necessary to definitively determine whether the theoretical benefits of leadless systems will be superior to those of conventional pacemakers both from a safety perspective (fewer acute and chronic complications) and in terms of long-term performance and efficacy. However, comparison to historical controls and claims data supports the concept that eliminating leads and the surgical pocket significantly reduces complications. As the technology progresses, it is possible that pacing leads will become extinct. Pacing therapy has overcome some of the initial hurdles and skepticism faced by Hayman. Leadless pacing offers the potential of antibradycardia support with markedly reduced complications. Future developments will include dual chamber leadless pacing, leadless cardiac resynchronization, and integration of leadless pacing with subcutaneous defibrillation.